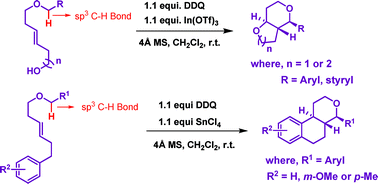
B. V. Subba Reddy et al. report the stereoselective synthesis of cis– and trans-fused bicyclic and tricyclic tetrahydropyran scaffolds. Their method involves DDQ oxidation to activate the benzylic C-H of various benzyl ethers and subsequent intramolecular Prins cyclization to give the dioxa-bicycles and oxatricycles.
Read the details for free for the next 4 weeks…..
Oxidative Prins and Prins/Friedel–Crafts cyclizations for the stereoselective synthesis of dioxabicycles and hexahydro-1H-benzo[f]isochromenes via the benzylic C–H activation
B. V. Subba Reddy, Prashant Borkar, J. S. Yadav, P. Purushotham Reddy, A. C. Kunwar, B. Sridhar and René Grée
DOI: 10.1039/C1OB06489D