Tooth and claw
Biological structures such as teeth and bone are produced by a process called biomineralisation. The shape and structure of biominerals are controlled in an extremely precise way, from the nanoscale right up to the macroscopic level. The spontaneous formation of a tooth, claw, spine or shell is a truly amazing piece of chemistry, and unlocking the secrets of this could prove vital for materials scientists in their quest to engineer ever more complex nanostructures.
Biomineralisation of calcium carbonate
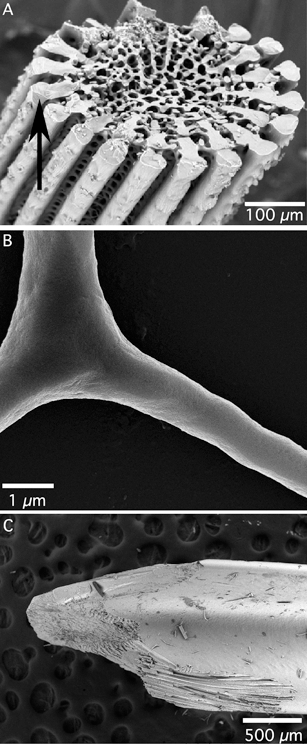
In Nanoscale, we have recently published some papers on the formation of crystalline calcium carbonate (calcite) by biomineralisation. Yang et al., in their paper entitled ‘Biomineral nanoparticles are space filling’, present a study of biomineralisation in sea urchins, which contain spicules, spines and teeth (pictured left) all composed of calcium carbonate. They discuss the formation of crystalline biominerals through amorphous precursors, where one can imagine hydrated amorphous calcium carbonate nanoparticulate building blocks being able to flow and morph into the intricate shape of the final biomineral, followed by a period of dehydration and crystallization which forms the solid product. This process of dehydration and crystallization is discussed further by Rodriguez-Blanco et al., who used time-resolved X-ray diffraction in order to study the changes in crystal structure which occur when amorphous calcium carbonate crystallizes. They discovered that, under certain conditions, crystallization from the amorphous form to calcite occurs via another crystalline form, known as vaterite.
In November’s themed issue of Nanoscale, entitled Crystallization and Formation Mechanisms of Nanostructures, we published some other work on calcium biominarlisation, including studies of the formation and stability of amorphous calcium carbonate by Jiang et al. and Sommerdijk et al., and of calcium phosphate crystals by Mann et al., Taubert et al., Zhai et al., and Ibsen and Birkedal, which discussed the use of structure directing agents and organic additives to control crystal growth and morphology.
Papers like these are prime examples of how the study of natural processes can provide vital insight into the synthetic mechanisms which scientists are developing to produce new nanomaterials.