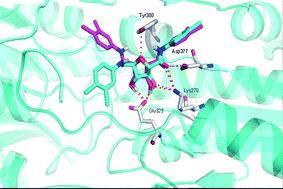
Phosphofructokinase is a kinase enzyme that phosphorylates fructose 6-phosphate in the glycolysis metabolic pathway, and is of central importance to carbohydrate metabolism. As such it is a very promising target for anti-trypanosomal drug design to treat sleeping sickness.
In this review Renata B. Oliveira et al. present a survey of recent literature regarding the structural and functional properties of phosphofructokinase as well as discussing its importance as a target in the development of selective therapeutics to treat Human African trypanosomiasis.
Phosphofructokinase: structural and functional aspects and design of selective inhibitors
Stefânia N. Lavorato, Saulo F. Andrade, Thaïs H. A. Silva, Ricardo J. Alves and Renata B. Oliveira
DOI: 10.1039/C2MD20122D












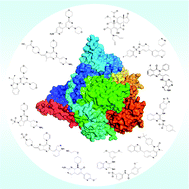 The phosphoinositide 3-kinase (PI3K) pathway is one of the most important signalling cascades in cancer. It has been well established as an attractive oncology target and inhibitors of PI3K have been suggested as promising agents for therapeutic intervention in cancer. Since the discovery of wortmannin and LY294002, the first compounds to inhibit PI3K, a vast number of inhibitors have been identified.
The phosphoinositide 3-kinase (PI3K) pathway is one of the most important signalling cascades in cancer. It has been well established as an attractive oncology target and inhibitors of PI3K have been suggested as promising agents for therapeutic intervention in cancer. Since the discovery of wortmannin and LY294002, the first compounds to inhibit PI3K, a vast number of inhibitors have been identified.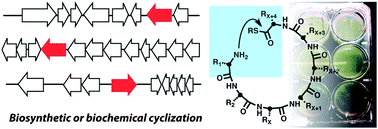 Albert A. Bowers
Albert A. Bowers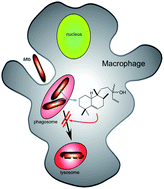
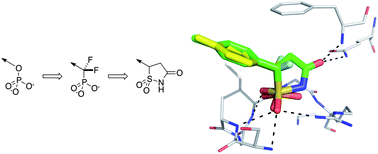 This review from
This review from 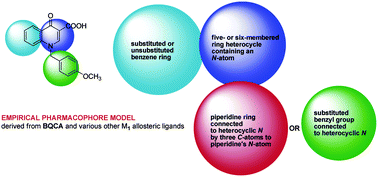
![GA[3]](https://blogs.rsc.org/md/files/2012/06/GA31.gif)
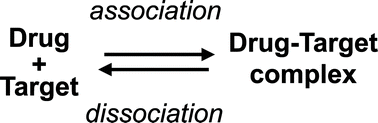 Previously drug–receptor interactions have only been quantified in terms of their affinity and efficacy but recently the residence time has also been recognized to affect the clinical performance.
Previously drug–receptor interactions have only been quantified in terms of their affinity and efficacy but recently the residence time has also been recognized to affect the clinical performance.