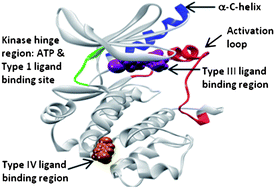
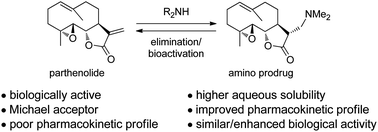
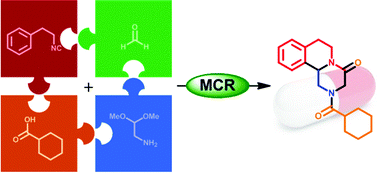
New drug design strategies are required that deliver agents possessing ‘small molecule properties’ but that also possess the ability to disrupt interactions between large protein surfaces in a similar manner to current protein-based therapeutics.
Macrocycle-based drug design represents a new and compelling strategy that could fulfil this need; however typical high-dilution macrocyclisation conditions are inefficient and impractical in a pharmaceutical setting from cost, capacity and green chemistry perspectives.
 In this review James C. Collins and Keith James from the Scripps Research Institute critically analyse macrocyclization reactions published over the last three years. Based upon on this analysis, and first-hand experience of macrocyclization, Collins and James propose a macrocyclization efficiency index, Emac, as a means of determining the true efficiency of a macrocyclization reaction.
In this review James C. Collins and Keith James from the Scripps Research Institute critically analyse macrocyclization reactions published over the last three years. Based upon on this analysis, and first-hand experience of macrocyclization, Collins and James propose a macrocyclization efficiency index, Emac, as a means of determining the true efficiency of a macrocyclization reaction.
Emac takes into account both reaction yield and concentration, which Collins & James state addresses the key factors that determine the practicality of using a given reaction in a drug discovery context. In other words, the Emac for a reaction indicates the likelihood of being able to conduct the reaction on a sufficiently large scale whilst remaining resource efficient.
This index also allows comparison of a large number of literature macrocyclization reactions and identifies those which deliver the powerful combination of high yield and high practicality. Collins and James hope that those who are actively engaged within the macrocyclization community will calculate the Emac for their reactions and report it in their publications, allowing the synthesis community to place the reaction within the context of the framework they outline in the review.
To read more about this index download the review today.
Emac – a comparative index for the assessment of macrocyclization efficiency
James C. Collins and Keith James
DOI: 10.1039/C2MD20176C
From the collection: The space in-between small molecules and biologicals












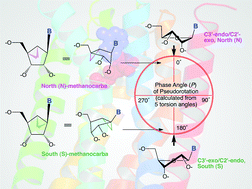

![GA[2]](https://blogs.rsc.org/md/files/2012/10/GA2.gif)
![GA[3]](https://blogs.rsc.org/md/files/2012/10/GA3.gif)