From space shuttles to military equipment, or even the kitchenware that we use on daily basis, lasers have found use in more places than we often realise. Interestingly, the solid-state lasers were believed to be a solution to an unknown problem after their invention in 1950s. In that time nobody—including their developer Charles Townes—noticed that the lasers were one of the game changer inventions in the world’s history. After tens of years, in 1964, the laser technology was awarded with a Nobel prize when its potential for diverse applications were realized.
So far we have only discussed solid-state lasers, but there is definitely capacity in the laser world to improve the performance and versatility with the help of little twists, such as optofluidic lasers. “Optofluidics” is a synergic combination of optical systems and microfluidics. In other words, optical systems are built or synthesized from liquids, aiming to serve as good as their solid-state equivalents. For example, two immiscible liquids form a smooth surface in their interface, leading to a laser cavity or an optical resonator with a very high Q-factor that allows for operating at low energy levels. This emerging field gains importance when considering most of the biochemical reactions that occur in aqueous environments. Optofluidic laser systems are flexible to change their optical properties by just replacing the liquid media; and with this twist, lasers have new application areas including diagnosis of genetic disorders at the cellular level and in vivo biosensing.
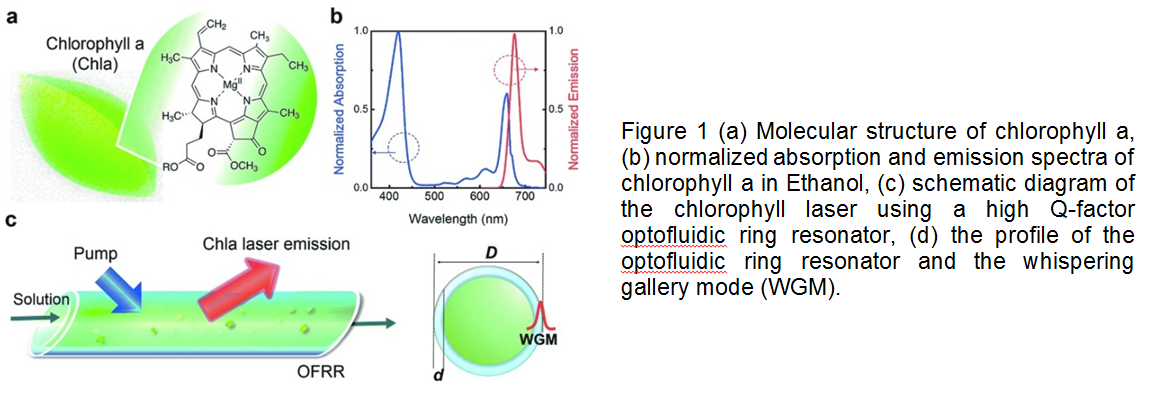
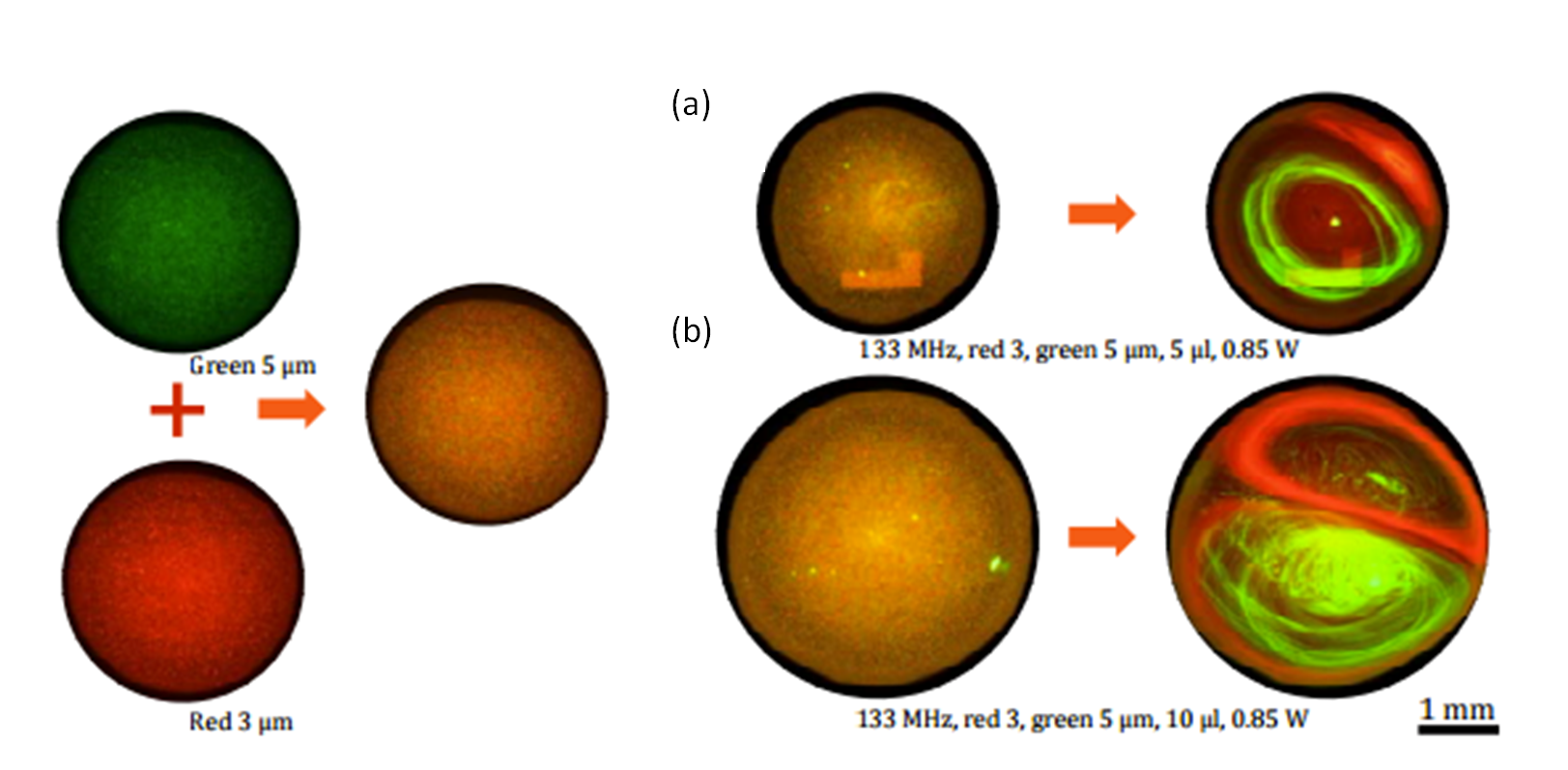
What is more exciting about the optofluidic lasers is that they can be biodegradable and easily tunable in microenvironments. Researchers in University of Michigan recently showed that one of the most abundant pigments on earth, chlorophylls, can maintain both biodegradability and tunability in optical systems owing to their fluorescence capabilities. Chlorophylls have a high Q-factor, dual-absorption bands in the visible spectrum, and a large shift between absorption and emission bands, suggesting that chlorophylls can be used as donors in fluorescence resonance energy transfer (FRET) laser (Figure 1). In this study, chlorophyll a was isolated from spinach leaf and used as the gain medium and the donor to develop a novel optofluidic laser. Two lasing bands of chlorophyll a was investigated by both theoretical and experimental means. Concentration-dependent studies enabled more insight for the mechanism determining when, where, and why the laser emission band appears. This new technique seems to gain increasing attention for applications in in vivo and in vitro biosensing, solar lighting and energy harvesting.
This article, published on 12th May 2016, is included in the Lab on a Chip Recent HOT Articles themed collection.
To download the full article for free* click the link below:
Optofluidic chlorophyll lasers
Yu-Cheng Chen, Qiushu Chena, Xudong Fan
Lab Chip, 2016, 16, 2228-2235
DOI: 10.1039/C6LC00512H

About the Webwriter
Burcu Gumuscu is a PhD researcher in BIOS Lab on a Chip Group at University of Twente in The Netherlands. Her research interests include development of microfluidic devices for second generation sequencing, organ-on-chip development, and desalination of water on the micron-scale.
*Access is free until 23/09/2016 through a registered RSC account.