This month sees the following articles in Lab on a Chip that are in the top ten most accessed:-
Cell lysis and DNA extraction of gram-positive and gram-negative bacteria from whole blood in a disposable microfluidic chip
Madhumita Mahalanabis, Hussam Al-Muayad, M. Dominika Kulinski, Dave Altman and Catherine M. Klapperich
Lab Chip, 2009, 9, 2811-2817, DOI: 10.1039/B905065P, Paper
Overview of single-cell analyses: microdevices and applications
Sara Lindström and Helene Andersson-Svahn
Lab Chip, 2010, 10, 3363-3372, DOI: 10.1039/C0LC00150C, Critical Review
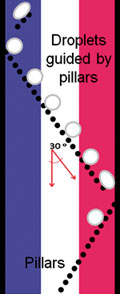
Rails and anchors: guiding and trapping droplet microreactors in two dimensions
Paul Abbyad, Rémi Dangla, Antigoni Alexandrou and Charles N. Baroud
Lab Chip, 2011, Advance Article, DOI: 10.1039/C0LC00104J, Paper
Fabrication of monolithic 3D micro-systems
Pakorn Preechaburana and Daniel Filippini
Lab Chip, 2011, 11, 288-295, DOI: 10.1039/C0LC00331J, Paper
Fully integrated lab-on-a-disc for simultaneous analysis of biochemistry and immunoassay from whole blood
Beom Seok Lee, Yang Ui Lee, Han-Sang Kim, Tae-Hyeong Kim, Jiwoon Park, Jeong-Gun Lee, Jintae Kim, Hanshin Kim, Wee Gyo Lee and Yoon-Kyoung Cho
Lab Chip, 2011, 11, 70-78, DOI: 10.1039/C0LC00205D, Paper
Design, engineering and utility of biotic games
Ingmar H. Riedel-Kruse, Alice M. Chung, Burak Dura, Andrea L. Hamilton and Byung C. Lee
Lab Chip, 2011, 11, 14-22, DOI: 10.1039/C0LC00399A, Paper
Cost-effective and compact wide-field fluorescent imaging on a cell-phone
Hongying Zhu, Oguzhan Yaglidere, Ting-Wei Su, Derek Tseng and Aydogan Ozcan
Lab Chip, 2011, 11, 315-322, DOI: 10.1039/C0LC00358A, Paper
A fast and simple method to fabricate circular microchannels in polydimethylsiloxane (PDMS)
Mohamed Abdelgawad, Chun Wu, Wei-Yin Chien, William R. Geddie, Michael A. S. Jewett and Yu Sun
Lab Chip, 2011, Advance Article, DOI: 10.1039/C0LC00093K, Technical Note
Three-dimensional microwell arrays for cell culture
Christina L. Randall, Yevgeniy V. Kalinin, Mustapha Jamal, Tanmay Manohar and David H. Gracias
Lab Chip, 2011, 11, 127-131, DOI: 10.1039/C0LC00368A, Communication
A microfluidic array with cellular valving for single cell co-culture
Jean-Philippe Frimat, Marco Becker, Ya-Yu Chiang, Ulrich Marggraf, Dirk Janasek, Jan G. Hengstler, Joachim Franzke and Jonathan West
Lab Chip, 2011, 11, 231-237, DOI: 10.1039/C0LC00172D, Paper
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.