To mark the occasion of Green Chemistry completing fifteen years of publication we invited contributions from authors who have had highly cited articles from each of the past 15 years. The result is a growing web collection covering topics of current importance in green chemistry from those who have contributed to developing the field. Details of the contributors, their highly cited article from the year they are representing, and their recent contribution are below.
Accompanying this collection and further celebrating ‘15 years of Green Chemistry’ is an Editorial containing contributions from all of Green Chemistry’s Chairs of the Editorial board and Scientific Editors giving their views on the area of green chemistry and the changes they have seen since the Journal was launched in 1999… read the Editorial here.
The 15 Years of Green Chemistry collection will be added to throughout 2014 and you can access the articles by clicking on the titles below, or look at the full collection of recent articles online here.
| Year | 15 Years of Green Chemistry Contribution | Original Highly Cited Article |
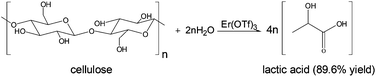
| 1999 | Journey on greener pathways: from the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation Rajender S. Varma, 2014, Perspective |
Solvent-free organic syntheses. using supported reagents and microwave irradiation, Rajender S. Varma, 1999, Paper |
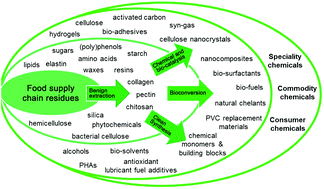
| 2000 | Food waste biomass: a resource for high-value chemicals Lucie A. Pfaltzgraff, Mario De bruyn, Emma C. Cooper, Vitaly Budarin and James H. Clark, 2013, Perspective |
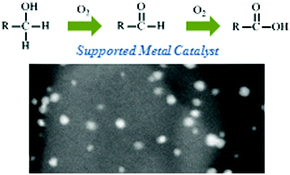
Preparation of a novel silica-supported palladium catalyst and its use in the Heck reaction James H. Clark, Duncan J. Macquarrie and Egid B. Mubofu, 2000, Paper |
| 2001 | Mixing ionic liquids – “simple mixtures” or “double salts”? Gregory Chatel, Jorge F. B. Pereira, Varun Debbeti, Hui Wang and Robin D. Rogers, 2014, Critical Review |
Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation, Jonathan G. Huddleston, Ann E. Visser, W. Matthew Reichert, Heather D. Willauer, Grant A. Broker and Robin D. Rogers, 2001, paper |
| 2005 | Green and sustainable manufacture of chemicals from biomass: state of the art Roger A. Sheldon, 2014, Critical Review |
Green solvents for sustainable organic synthesis: state of the art Roger A. Sheldon, 2005, Critical Review |
| 2006 | Are ionic liquids a proper solution to current environmental challenges? Giorgio Cevasco and Cinzia Chiappe, 2014, Critical Review |
Acute toxicity of ionic liquids to the zebrafish (Danio rerio) Carlo Pretti, Cinzia Chiappe, Daniela Pieraccini, Michela Gregori, Francesca Abramo, Gianfranca Monni and Luigi Intorre, 2006, Communication |
| 2007 | Pharmaceutical Green Chemistry process changes – how long does it take to obtain regulatory approval? Peter J. Dunn, 2013, Perspective |
Key green chemistry research areas—a perspective from pharmaceutical manufacturers David J. C. Constable, Peter J. Dunn, John D. Hayler, Guy R. Humphrey, Johnnie L. Leazer, Jr., Russell J. Linderman, Kurt Lorenz, Julie Manley, Bruce A. Pearlman, Andrew Wells, Aleksey Zaks and Tony Y. Zhang, 2007, Perspective |
| 2008 | Towards resource efficient chemistry: Tandem reactions with renewables Arno Behr, Andreas Johannes Vorholt, Thomas Seidensticker and Karoline Anna Ostrowski, 2013, Critical Review |
Improved utilisation of renewable resources: New important derivatives of glycerol Arno Behr, Jens Eilting, Ken Irawadi, Julia Leschinski and Falk Lindner, 2008, Critical Review |
| 2009 | Conversion of glucose and cellulose into value-added products in water and ionic liquids Jinliang Song, Honglei Fan, Jun Ma and Buxing, 2013, Tutorial Review |
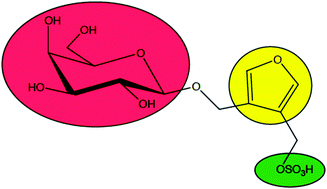
Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid Suqin Hu, Zhaofu Zhang, Jinliang Song, Yinxi Zhou and Buxing Han, 2009, Communication |
| 2011 | Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels Maria J. Climent, Avelino Corma and Sara Iborra, 2014, Critical Review |
Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts Maria J. Climent, Avelino Corma and Sara Iborra, 2011, Critical Review |
| 2012 | Continuous process technology: a tool for sustainable production Charlotte Wiles and Paul Watts, 2014, Tutorial Review |
Continuous flow reactors: a perspective Charlotte Wiles and Paul Watts, 2012, Tutorial Review |











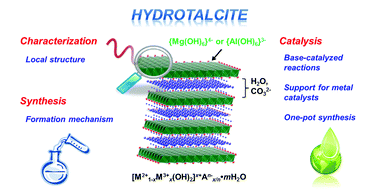
 As the year draws to a close, here is a list of the top 10 cited review articles in Green Chemistry in 2012 – all free to access until the
As the year draws to a close, here is a list of the top 10 cited review articles in Green Chemistry in 2012 – all free to access until the