
Professor Martyn Poliakoff
Green Chemistry is delighted to announce that Professor Martyn Poliakoff has been awarded a Knighthood in the New Year’s Honours list.
Professor Poliakoff is closely associated with Green Chemistry and was Chair of the Editorial Board from 2006–2012. He says, “I have been associated with Green Chemistry almost from the outset, and I continue to regard it as the leading journal in the field. Long may it continue!”
Professor Poliakoff is an inorganic chemist, whose work has been taken up by industry, notably in the construction of the world’s first multi-reaction supercritical fluid plant. He is a global leader in the field of green and sustainable chemistry and his work in engaging the public with chemistry has been recognised internationally, including through the Periodic Table of Videos on YouTube. Make sure to take a look at the special video made recently to announce the knighthood for Professor Polaikoff online here. Shortly before Christmas he was also elected an Honorary Fellow of the Royal Society of Chemistry.
Professor Poliakoff said “I feel both honoured and somewhat overwhelmed. I see the award very much as recognition of all the work being done in green and sustainable chemistry in the School of Chemistry by my colleagues, by my research team and by our technical staff whose efforts underpin so much of our research.”
Professor Sir David Greenaway, Vice-Chancellor of The University of Nottingham, said: “What a richly deserved accolade. Sir Martyn contributes so much as a research leader, educator and communicator of science to the wider public. He will receive this honour with his customary modesty, but will be surprised at how widely applauded it is. We are very proud to have him as a colleague.”

Professor Kenneth Seddon
Professor Kenneth Seddon, Green Chemistry Advisory Board member, has also been honoured in the New Year’s Honours list with an OBE for services to Chemistry.
In addition to his ground-breaking research into ionic liquids Professor Seddon has also been very busy recently, having been involved with a featured exhibit at the Royal Society Summer Exhibition, presenting the Inaugural Lecture of the “Frontiers of Knowledge Lecture Series”, House of Commons, receiving four IChemE awards, the Nicklin medal, and the RSC Teamwork award.
The New Year Honours lists recognise the achievements of a wide range of extraordinary people across the UK, you can read more about them here.
Take a look at some recent contributions to Green Chemistry from Martyn Poliakoff and Kenneth Seddon. These are all free to access until the end of February.
15 years of Green Chemistry
James Clark, Roger Sheldon, Colin Raston, Martyn Poliakoff and Walter Leitner
DOI: 10.1039/C3GC90047A, Editorial
Synthesis of metal–organic frameworks by continuous flow
Peter A. Bayliss, Ilich A. Ibarra, Eduardo Pérez, Sihai Yang, Chiu C. Tang, Martyn Poliakoff and Martin Schröder
DOI: 10.1039/C4GC00313F, Paper
Synthesis of antimalarial trioxanes via continuous photo-oxidation with 1O2 in supercritical CO2
Jessica F. B. Hall, Richard A. Bourne, Xue Han, James H. Earley, Martyn Poliakoff and Michael W. George
DOI: 10.1039/C2GC36711D, Paper
Enhanced laccase stability through mediator partitioning into hydrophobic ionic liquids
Lars Rehmann, Ekaterina Ivanova, H. Q. Nimal Gunaratne, Kenneth R. Seddon and Gill Stephens
DOI: 10.1039/C3GC42189A, Paper
Tailoring ionic liquid catalysts: structure, acidity and catalytic activity of protonic ionic liquids based on anionic clusters, [(HSO4)(H2SO4)x]− (x = 0, 1, or 2)
Karolina Matuszek, Anna Chrobok, Fergal Coleman, Kenneth R. Seddon and Małgorzata Swadźba-Kwaśny
DOI: 10.1039/C4GC00415A, Paper
















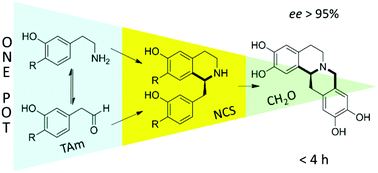 Open access communication: One-pot triangular chemoenzymatic cascades for the synthesis of chiral alkaloids from dopamine
Open access communication: One-pot triangular chemoenzymatic cascades for the synthesis of chiral alkaloids from dopamine