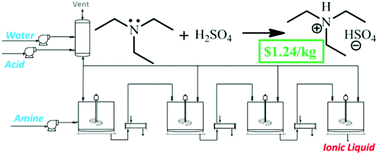
In this latest work from Jason Hallett and colleagues from Imperial College London, the economic feasibility of two ionic liquids synthesized by acid–base neutralization has been assessed. It was found that process intensification dramatically reduces the end cost of these ionic liquids, and is recommended in this latest work as a means of reducing the cost of ionic liquids so that their potential in commercial applications may be realised.
The prices of triethylammonium hydrogen sulfate and 1-methylimidazolium hydrogen sulfate produced with optimised manufacturing methods are estimated to be as little as $1.24 kg−1 and $2.96 kg−1 respectively, which are largely dictated by the raw material costs. These prices are similar to conventional organic solvents such as acetone, while at present typical ionic liquid prices can be two orders of magnitude greater than this. The authors conclude that more effort should be dedicated to developing new ionic liquids that can be synthesised from affordable raw materials in very few steps.
Inexpensive ionic liquids: [HSO4]−-based solvent production at bulk scale
L. Chen et al., Green Chem., 2014. DOI: 10.1039/C4GC00016A
http://pubs.rsc.org/en/content/articlelanding/2014/gc/c4gc00016a#!divAbstract










