The 2014 Great Lakes Green Chemistry Conference – Innovating for Success in the Great Lakes Region – is taking place on April 1-2, 2014 in Cleveland, OH and there’s still time to register. This two-day conference will bring together businesses, researchers, state environmental agencies and NGOs to hear about how Green Chemistry and sustainable chemistry initiatives in the Great Lakes are driving innovations all along the supply chain. Keynote speakers include some of the most prominent names in the field of Green Chemistry. Our morning keynote John Warner is the co-author of the landmark publication Green Chemistry, Theory and Practice, and is currently the president and chief technology officer at Warner Babcock Institute for Green Chemistry. He will talk about “Mechanisms from Nature in Materials Design”. Julie Zimmerman, our luncheon keynote, is the Associate Director of the Center for Green Chemistry and Green Engineering at Yale University, will speak about “Green Engineering: Fundamentals, Sustainability, and Design”, and our final keynote on Tuesday morning will be Dennis McGavis, Global Director of EHS Sustainability at Goodyear Rubber & Tire Co. Dennis will be presenting on “Two big challenges for green chemistry innovation – from an industry perspective”.

The conference will also include several dynamic panels and breakout sessions with opportunities to hear from companies on the cutting edge of innovation in the region, as well as from academic institutions such as the University of Toledo who has just opened their new School of Green Chemistry and Engineering. Panels will address the Imperative for Green Chemistry in the Great Lakes, How Policy Drives Green Chemistry Innovation,: The Business Perspective, Green Chemistry Innovations in Business, and Getting Started on Alternatives Assessments, followed by breakout sessions where participants will have the opportunity to engage in more in-depth discussion on issues raised with the panel sessions.
On April 1, the first night of the conference, the Great Lakes Green Chemistry Network, one of the conference sponsors, will host a dinner at the Mallorca Restaurant in Cleveland in honor of John Warner, to which all conference registrants are invited to make reservations.
More information and registration for the conference can be found at www.glrppr.org/conference/registration
Two additional events will also be available as part of the conference. On March 31, the Great Lakes Regional Pollution Prevention Roundtable will hold a half day meeting. Separate registration is required and more information is available from at Laura Barnes at l-barnes@illinois.edu. Immediately following the conference, on Thursday, April 3, there will be a one-day GreenScreen™ for Safer Chemicals Training. This is a training on the hazard assessment tool, GreenScreen™ for Safer Chemicals, developed by Clean Production Action for identifying chemicals of concern and selecting safer alternatives. Separate registration is required, and more information is available by contacting greenscreen@cleanproduction.org














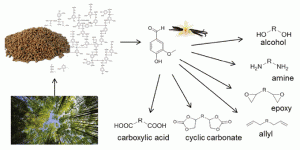
 Shrimp shells that would otherwise be thrown away by the seafood industry have been turned into
Shrimp shells that would otherwise be thrown away by the seafood industry have been turned into  The outside front cover this month (pictured left) features work by Javier Pérez-Ramírez and co-workers from Zurich, Switzerland. In their work they report how the Lewis-acid catalysed isomerisation of bio-oil derived glyocal over tin-based zeolites efficiently and sustainably produces glycolic acid and alykyl glycolates.
The outside front cover this month (pictured left) features work by Javier Pérez-Ramírez and co-workers from Zurich, Switzerland. In their work they report how the Lewis-acid catalysed isomerisation of bio-oil derived glyocal over tin-based zeolites efficiently and sustainably produces glycolic acid and alykyl glycolates. The inside front cover this month (pictured right) features work by Philip Jessop and co-workers from Ontario, Canada. In their work they focus on switchable-hydrophilicity solvents (SHS), which can switch reversibly between one form that is miscible with water and another that forms a biphasic mixture with water. They report new examples and compare them in terms of safety and environmental impact.
The inside front cover this month (pictured right) features work by Philip Jessop and co-workers from Ontario, Canada. In their work they focus on switchable-hydrophilicity solvents (SHS), which can switch reversibly between one form that is miscible with water and another that forms a biphasic mixture with water. They report new examples and compare them in terms of safety and environmental impact.