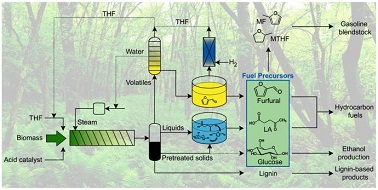
Charles Wyman and colleagues have demonstrated a highly effective lignocellulosic bio-refinery model, producing unrivalled quantities of furfural, 5-hydroxymethylfurfural (HMF), and levulinic acid as products. Proposed as a basis for the production of renewable fuels, the furan derivatives generated by this process are also a promising platform onto many other intriguing bio-based molecules. Additionally, more than 90% of the lignin contained within the maple wood feedstock was reclaimed, making the process as a whole exceptionally appealing.
![GA[3]](https://blogs.rsc.org/gc/files/2013/09/GA3.gif) Instead of the typical acidified aqueous media often used, the hydrolysis of the lignocellulose feedstock (and subsequent sugar dehydration) was enhanced with the addition of the potentially renewable solvent tetrahydrofuran. The organic solvent increased yields of HMF by an order of magnitude over what could be obtained otherwise in a one-pot reaction conducted at 170 °C. Under these optimised conditions, 87% of the total pentose (C5) sugar content was accountable in the form of furfural, while the hexose (C6) sugar content was converted from cellulose into HMF (in up to yields of 21% of the theoretical maximum), levulinic acid (up to 40%), and free sugars. The authors believe this represents a key step towards achieving commercial viability for sustainable, sugar derived bio-fuels.
Instead of the typical acidified aqueous media often used, the hydrolysis of the lignocellulose feedstock (and subsequent sugar dehydration) was enhanced with the addition of the potentially renewable solvent tetrahydrofuran. The organic solvent increased yields of HMF by an order of magnitude over what could be obtained otherwise in a one-pot reaction conducted at 170 °C. Under these optimised conditions, 87% of the total pentose (C5) sugar content was accountable in the form of furfural, while the hexose (C6) sugar content was converted from cellulose into HMF (in up to yields of 21% of the theoretical maximum), levulinic acid (up to 40%), and free sugars. The authors believe this represents a key step towards achieving commercial viability for sustainable, sugar derived bio-fuels.
By James Sherwood
This article is free to access for 4 weeks! Click the link below to read more:
THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass, Charles M. Cai, Taiying Zhang, Rajeev Kumar and Charles E. Wyman, Green Chem., 2013, DOI: 10.1039/c3gc41214h














![GA[1]](https://blogs.rsc.org/gc/files/2013/09/GA1.gif)
![GA[2]](https://blogs.rsc.org/gc/files/2013/09/GA2.gif)