The latest issue of Green Chemistry is now available to read online.
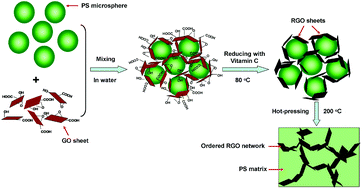
 The front cover of this issue highlights a Critical Review by Tom Welton and colleagues from Imperial College London (UK) and Umeå University (Sweden) on the deconstruction of lignocellulosic biomass with ionic liquids. The review begins by providing background information on ionic liquids and lignocellulosic biomass before going on to explore the solubility of lignocellulosic biomass in ionic liquids. The also describes the destruction effects brought about by the use of ionic liquids as a solvent, before finally looking at the practical considerations for design of ionic liquid based deconstruction processes.
The front cover of this issue highlights a Critical Review by Tom Welton and colleagues from Imperial College London (UK) and Umeå University (Sweden) on the deconstruction of lignocellulosic biomass with ionic liquids. The review begins by providing background information on ionic liquids and lignocellulosic biomass before going on to explore the solubility of lignocellulosic biomass in ionic liquids. The also describes the destruction effects brought about by the use of ionic liquids as a solvent, before finally looking at the practical considerations for design of ionic liquid based deconstruction processes.
Deconstruction of lignocellulosic biomass with ionic liquids, Agnieszka Brandt, John Gräsvik, Jason P. Hallett and Tom Welton, Green Chem., 2013, 15, 550-583
 The inside front cover features work by Robert Brown and Kaige Wang from Iowa State University, USA, who report the catalytic pyrolysis of microalgae for production of valuable petrochemicals and ammonia. This promising microalgae biorefinery pathway (both from an economical and environmental point-of-view) used the HZSM-5 catalyst for pyrolysis to convert whole microalgae into aromatic hydrocarbons. The ammonia produced in the process can be recycled as a fertilizer for microalgae cultivation.
The inside front cover features work by Robert Brown and Kaige Wang from Iowa State University, USA, who report the catalytic pyrolysis of microalgae for production of valuable petrochemicals and ammonia. This promising microalgae biorefinery pathway (both from an economical and environmental point-of-view) used the HZSM-5 catalyst for pyrolysis to convert whole microalgae into aromatic hydrocarbons. The ammonia produced in the process can be recycled as a fertilizer for microalgae cultivation.
Catalytic pyrolysis of microalgae for production of aromatics and ammonia, Kaige Wang and Robert C. Brown, Green Chem., 2013, 15, 675-681
These articles are free to access for 6 weeks!
Keep up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.