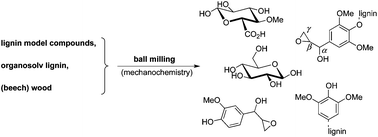
Biocatalysis is becoming an increasingly attractive method to achieve enantiopure chemicals, in a cleaner and more environmentally sustainable way. This is in part due to the fact that in most of these reactions, water is used as the solvent primarily because the enzyme catalysts are most stable in this medium. However, some substrates are insoluble or only sparingly soluble in water which can limit the use of these enzymes.
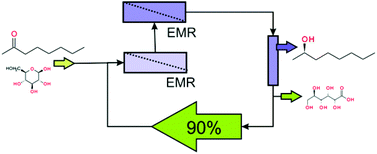
Finally, downstream adsorption of the resulting alcohols allowed 90% recycling of the aqueous buffer solution, reducing the E-factor of the process to 13.
This article is currently free to access until the 3rd January 2013!
Enantioselective reduction of sparingly water-soluble ketones: continuous process and recycle of the aqueous buffer system, Susanne Leuchs, Shukrallah Na’amnieh and Lasse Greiner, Green Chem., 2013, DOI: 10.1039/C2GC36558H
You may also be interested in these articles as well – free to access for 2 weeks:
Utilising hardly-water soluble substrates as a second phase enables the straightforward synthesis of chiral alcohols, Christina Kohlmann, Nora Robertz, Susanne Leuchs, Lasse Greiner and Shukralla Na’amnieh, Green Chem., 2011, 13, 3093-3095
Continuous biocatalytic synthesis of (R)-2-octanol with integrated product separation, Christina Kohlmann, Susanne Leuchs, Lasse Greiner and Walter Leitner, Green Chem., 2011, 13, 1430-1436
Stay up-to-date with the latest news and content in Green Chemistry by registering for our free table of contents alerts.