A quick bench-top solvent guide reference has been developed in order for alternative solvents to dichloromethane (DCM) to be selected for separation of a variety of organic molecules.
Chromatography is widely used by synthetic chemists for purification as it can be broadly applied to a vast range of compounds and is very adaptable. However, the largest contributor of chlorinated solvent waste in the medicinal chemistry industry is chromatography – primarily DCM. Given the significant human and environmental toxicities associated with DCM, reduction or ideally replacement of this solvent is important.
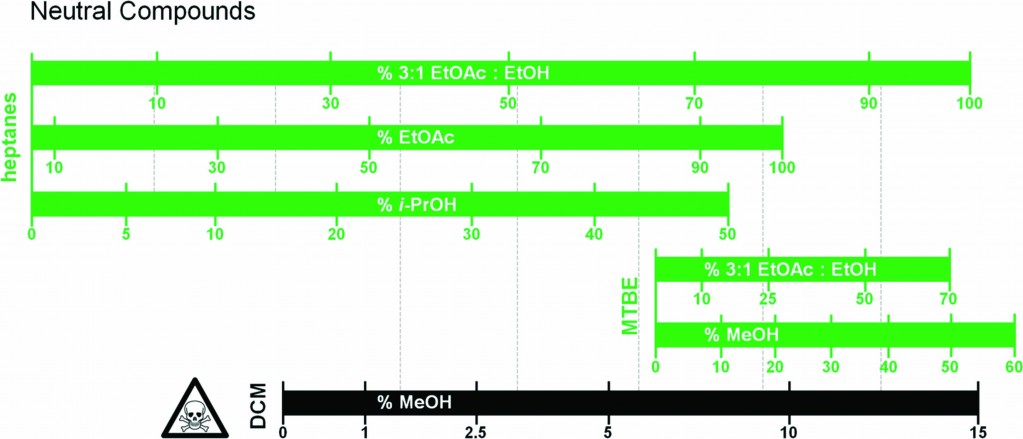 Here, Joshua Taygerly, Emily Peterson and colleagues from Amgen Inc. and Northeastern University, USA have developed a guide which aims to help synthetic chemists find suitable and more environmentally friendly alternatives to a DCM-solvent system for chromatographic purification of compounds. The authors selected several ‘drug-like’ molecules which reflected the types of molecules regularly prepared and purified, and separated these into three categories – acidic, basic and neutral (where ‘neutral’ refers to compounds without a carboxylic acid or amine functionality). They tested several alternative solvent systems and assembled a figure which allows the scientist to find the DCM solvent system that would have been applied to a particular molecule and follow it up vertically to find potentially equivalent systems (see the guide for neutral compounds right).
Here, Joshua Taygerly, Emily Peterson and colleagues from Amgen Inc. and Northeastern University, USA have developed a guide which aims to help synthetic chemists find suitable and more environmentally friendly alternatives to a DCM-solvent system for chromatographic purification of compounds. The authors selected several ‘drug-like’ molecules which reflected the types of molecules regularly prepared and purified, and separated these into three categories – acidic, basic and neutral (where ‘neutral’ refers to compounds without a carboxylic acid or amine functionality). They tested several alternative solvent systems and assembled a figure which allows the scientist to find the DCM solvent system that would have been applied to a particular molecule and follow it up vertically to find potentially equivalent systems (see the guide for neutral compounds right).
The primary use of this guide is to provide chemists with a quickly identifiable starting point for selecting alternative solvent systems to DCM.
You can read this article for free until the 17th October 2012!
A convenient guide to help select replacement solvents for dichloromethane in chromatography, Joshua P. Taygerly, Larry M. Miller, Alicia Yee and Emily A. Peterson, Green Chem., 2012, DOI: 10.1039/C2GC36064K
You may also be interested in these articles relating to solvent selection – free to access for 2 weeks:
Searching for green solvents, Philip G. Jessop, Green Chem., 2011, 13, 1391-1398
Expanding GSK’s solvent selection guide – embedding sustainability into solvent selection starting at medicinal chemistry, Richard K. Henderson, Concepción Jiménez-González, David J. C. Constable, Sarah R. Alston, Graham G. A. Inglis, Gail Fisher, James Sherwood, Steve P. Binks and Alan D. Curzons, Green Chem., 2011, 13, 854-862
Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation, Kim Alfonsi, Juan Colberg, Peter J. Dunn, Thomas Fevig, Sandra Jennings, Timothy A. Johnson, H. Peter Kleine, Craig Knight, Mark A. Nagy, David A. Perry and Mark Stefaniak, Green Chem., 2008, 10, 31-36
Stay up-to-date with the latest news and content in Green Chemistry by registering for our free table of contents alerts.










