| Recyclable mesoporous silica-supported chiral ruthenium-(NHC)NN-pincer catalysts for asymmetric reactions. Scientists from Spain have reported two new stable chiral Ru-pincer complexes with a pendant silyloxy group, which were grafted onto a mesoporous silica (MCM-41). These supported complexes were shown to be highly active and recyclable catalysts for the asymmetric hydrogenation of alkenes and the cyclopropanation of styrenes. These catalysts avoid the draw-backs associated with homogeneous catalysts such as high catalyst loadings and difficulties in catalyst recovery. The supported catalysts reported here showed no deactivation after repeated recycling. (Green Chem., 2011, DOI 10.1039/c1gc15412e) | 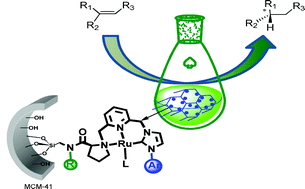 |
These articles are free to access until September 9th!










