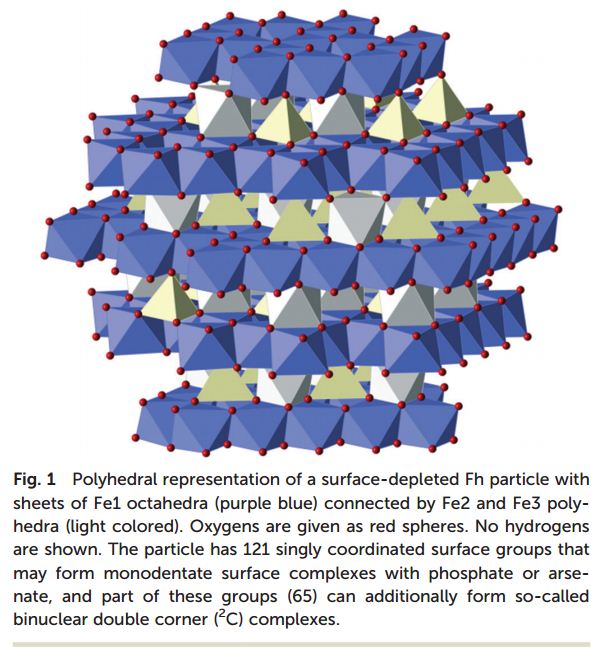
Iron oxide nanoparticles are not just a laboratory curiosity but a major presence within the whole of the natural world. Ferrihydrite is the predominant iron oxide nanoparticle found under abiotic conditions owing to its very low surface energy. The essential function of iron storage and transport within living organisms is carried out by the protein ferritin, made possible through the incorporation of iron nanoparticles inside the protein’s internal cavity.
The ability of ferritin to template the formation of iron oxide nanoparticles of defined size has been exploited in the production of systems used in targeted drug delivery, magnetic resonance imaging and nano-electronics. The ability of the iron core in ferritin to adsorb phosphate anions has also been put to use in removing this nutrient down to levels which prevent the development of bacteria on water purification membranes.
Understanding nanoparticles
In spite of being recognised for their essential function and with much work dedicated to the development of exciting applications surrounding them, iron oxide nanoparticles have been so far poorly understood at a fundamental level regarding their structure and reactivity models. Their implicitly small size and low symmetry has made imaging difficult using conventional crystallographic techniques. The properties of nanoparticles are generally dependent on their size and any model attempting to quantify the reactivity displayed by the surface must take this into account. The nature of the surface itself is dependent on the chemical properties of the surrounding environment.
Hiemstra and Zhao have conducted both an experimental and a computational study in order to generate a valid reactivity model for the adsorption of phosphate and arsenate by ferrihydrite and by the ferritin core. Ferrihydrite was modelled and essential properties such as surface area, density of surface reactive groups such as O(H), and surface charge were calculated as a function of the particle size.
The experimental study followed the adsorption of phosphate anions onto freshly prepared ferrihydrite and the effect of phosphate concentration on the formation and properties of iron oxide nanoparticles inside ferritin was analysed. Corroboration of theoretical with experimental data allowed for the development of an anion adsorption model with account for surface reactivity and generated new understanding concerning the formation, growth and aggregation of iron oxide nanoparticles under conditions relevant to environmental applications.
The full article is free to access* for a limited time only:
Tjisse Hiemstra and Wei Zhao
Environ. Sci.: Nano, 2016, Advance Article
DOI: 10.1039/C6EN00061D
About the webwriter

Dan Mercea is a PhD student in the Fuchter group at Imperial College London. He is working on developing enantioselective FLP catalysis.
—————-
*Access is free until 11th October 2016 through a registered RSC account – register here