Who doesn’t like power? We all do. That’s why we love modifying nanomaterial surfaces. It gives us the power to control their properties. In the case of Fe nanoparticles (Fe NP), which are heavily used in environmental remediation, partial deposition of secondary metals such as Pd, Ni, Cu or Pt results in bimetallic clusters with enhanced reaction efficiencies towards treating contaminated groundwater and soils. Nevertheless one has to be cautious about how these modifications affect the nanoparticle toxicity. In fact, Fe NPs are known to possess antibacterial and antifungal properties; potential mechanisms of toxicity are membrane disruption and oxidative damage from the reactive oxygen species (ROS).
Therefore, E-J. Kim and colleagues from the Pohang University of Science and Technology have investigated the mechanism of toxicity for 4 different bimetallic Fe NPs: Fe/Cu, Fe/Ni, Fe/Pd and Fe/Pt, toward Escherichia coli along with bare Fe NPs. Synthesis of NPs was completed in-house and characterization data showed 50-70 nm primary particles with homogenous secondary metal coatings and zerovalent Fe cores.
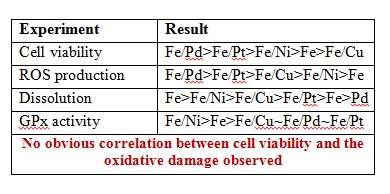
The initial experiments consisted of testing cell viability upon exposure to NPs using CFU assay, ROS production using DCF-DA fluorescence dye (3hr post exposure), peroxidase activity using antioxidant enzyme glutathione peroxidase (GPx) and NP dissolution to determine the role of oxidative stress on the cell death. The results obtained are summarized below.
Therefore, the team concluded an alternative mechanism of toxicity via membrane disruption using a spectroscopic approach with FTIR spectroscopy, TEM imaging and anion release profiles. The TEM images showed higher levels of uptake for Fe/Cu in contrast to Fe/Pd. FTIR spectra of bacterial cells showed peaks corresponding to the C-O/C-O-C, PO2– and CH3– stretches that disappear upon exposure to NPs. Finally, the anion release profiles were found to be the most consistent with the cell viability indicating that NP-mediated membrane permeability and/or membrane damage is the major mechanism of toxicity. Higher amounts of PO32- and Cl– were observed for cell cultures exposed to Fe/Cu while they were lowest for Fe/Pd and Fe/Pt. Interestingly Fe/Ni caused the highest release of SO42- indicating the disruption of sulfate transport system.
Overall, Fe/Pd was concluded to have the least toxicity and superior performance in terms of environmental decontamination while Fe/Cu NPs remained at the other end of the spectrum. The authors suggest this work may provide a platform for environmental engineers to design treatment strategies for environmental remediation with less harmful side effects over unmodified Fe NPs.
To read more about the full article, download a copy for free* by clicking the link below:
Comparative toxicity of bimetallic Fe nanoparticles toward Escherichia coli: mechanism and environmental implications
Eun-Ju Kim, Thao Le Thanh and Yoon-Seok Chang
DOI: 10.1039/C3EN00057E
*Access is free through a registered RSC account – click here to register