We have used nuclear energy for a while now. It is a clean form of energy, except for one little thing: what happens with radioactive waste? Scientists think the best solution is burying it deep in the ground and labelling it clearly enough so that future generations (or aliens!) will not dare to look inside. However, is this really the best solution? What happens with radioactive nuclei once they are inside the nuclear graveyard?
Scientists need to study the interactions of radioactive elements with the environment that surrounds them in the ground. But using radioactive elements is tricky: they can be dangerous and unstable, and most of them tend to decay in a few seconds (minutes, if you are lucky). Hence, researchers have determined to use models that mimic the behaviour of elements such as americium, curium or plutonium. Right in the row above actinides we find lanthanides, which have very similar oxidation states and comportment.
Scientists dig into europium. Not only because of its stability, but also because of its high fluorescence. This makes europium easy to track down in the lab. Outside the lab, europium is also very useful: the European Central Bank (ECB) uses europium as a fluorescent marker to fight counterfeit banknotes. Rumour has it the ECB intended the euro pun when choosing this particular element.
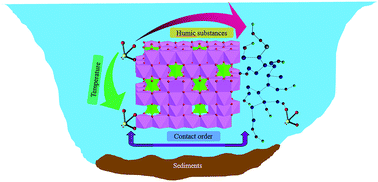
A group of researchers in China have studied the interactions of europium with alumina and humic acid (HA). These two substances represented the average inorganic and organic components of soil. In previous studies, they investigated the effect of reaction time, pH or ionic strength. In this paper, recently published in Environmental Science: Processes & Impacts, researchers examined the influence of temperature in the interactions of europium. And temperature is important when it comes to radioactive wastes: nuclear debris can keep temperatures of up to 100ºC during at least 1000 years, due to exothermic radioactive effects such as decomposition.
Luckily, the results were quite positive. Apparently, at high temperatures the formation of very stable structures is favoured, and the sorption of europium in alumina and alumina/HA systems is slightly increased with temperature. Nonetheless, trivalent cations are not the only substances present in nuclear waste. The interactions between soil-like substances (like alumina or HA) and other type of nuclei remain to be studied in depth.
Click on the link below to read the full article for free*
Sequestration and speciation of Eu(III) on gamma alumina: role of temperature and contact order
Yawen Cai, Xuemei Ren, Yue Lang, Zhiyong Liu, Pengfei Zong, Xiangke Wanga and Shitong Yang
Environ. Sci.: Processes Impacts, 2015, 17, 1904-1914
DOI: 10.1039/C5EM00412H
—————-
Fernando Gomollón-Bel is a PhD Student at the ISQCH (CSIC–University of Zaragoza). His research focuses on asymmetric organic synthesis using sugars as chiral-pool starting materials towards the production of fungical transglycosidase inhibitors.
—————-
* Access is free until 20/12/2015 through a registered RSC account.