Breakthrough for bacterial hydrogen production
by Philippa Ross
Scientists in China have developed a device that can produce hydrogen from organic materials using bacteria at temperatures below 25 degrees Celsius.
Normally, hydrogen production by bacterial metabolism is reduced at lower temperatures because it slows down the enzymes that catalyse the reactions. Now, Defeng Xing and his team at the Harbin Institute of Technology have optimised hydrogen production from organic matter between 4 and 9 degrees Celsius by using a microbial electrolysis cell (MEC). This eliminates the cost of heating and could enable hydrogen production to be carried out at high latitudes and mountainous regions where the air temperature is below 10 degrees Celsius.
MECs generate hydrogen directly upon applying an electric current to bacteria. Bacteria consume acetic acid, which is produced from fermenting plant matter and release protons, electrons and CO2. Addition of an electric current enables the protons and electrons to join together to make hydrogen gas and the higher the current, the more hydrogen is produced.
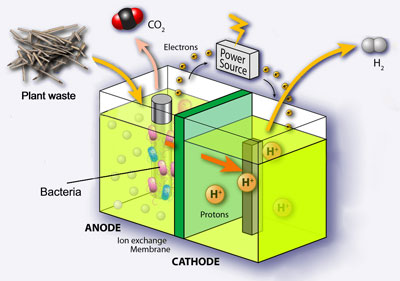
‘In order to achieve a high yield of hydrogen in MECs, it’s essential that both the electron transfer and hydrogen recovery processes are highly efficient,’ explains Xing.
Methanogenesis, or methane formation, is a common problem in MECs, which occurs at higher temperatures as a result of bacterial anaerobic respiration. This can reduce the efficiency of electron transfer to the cathode, reducing the overall output of hydrogen. However, at temperatures below 10 degrees Celsius, no methane was produced since the growth of the methane producing organisms was inhibited and the yield of hydrogen produced is comparable to that at temperatures above 25 degrees Celsius.
Sarah Strycharz-Glaven, an expert in microbial fuel cells at the Naval Research Laboratory in Washington DC is impressed with the group’s findings but acknowledges that there is some progress to be made. ‘The authors will need to increase the efficiency of hydrogen production under colder conditions to compete with MECs operating at ambient conditions,’ she says.
The group aim to do this by increasing hydrogen recovery and exploring new electrode materials. In the future they hope that MEC technology could be considered for biohydrogen production in cold environments.
Read the Energy & Environmental Science article now:
Hydrogen production, methanogen inhibition and microbial community structures in psychrophilic single-chamber microbial electrolysis cells
Lu Lu, Nanqi Ren, Xin Zhao, Huan Wang, Di Wu and Defeng Xing
Energy Environ. Sci., 2011, Advance Article, DOI: 10.1039/C0EE00588F
More Chemistry World stories
Comments Off on Breakthrough for bacterial hydrogen production











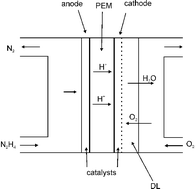 Reviewing recent advances in ammonia and hydrazine based electrochemical fuel cells
Reviewing recent advances in ammonia and hydrazine based electrochemical fuel cells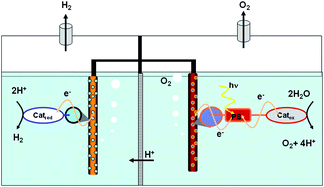 This review covers the progress achieved in the synthesis and characterization of different metal based catalysts designed for the photocatalytic oxidation of water, with special focus on molecular designed systems.
This review covers the progress achieved in the synthesis and characterization of different metal based catalysts designed for the photocatalytic oxidation of water, with special focus on molecular designed systems.