A new type of blue energy harvesting device may offer a practical method of continuous coastal electricity generation.
In 2011, Stanford researchers described a new form of energy harvesting device coined as a “mixing entropy battery” in Nano Letters1. Their device capitalized on the chemical energy available in a system where an ion concentration gradient is present; in this case where low salinity wastewater or river water was mixed with ~0.6 M NaCl rich seawater. It is estimated that during this mixing process, there is a free energy reduction of 2.2 kJ per liter of freshwater.
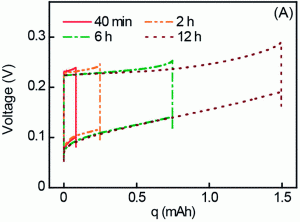
Using two electrodes, one Na+ selective the other Cl– selective, a charging state exists when the battery is exposed to low salinity water, where ions in the electrodes are removed via a concentration gradient. Next, sea water replaces the low salinity water and the potential between the electrodes increases. This is followed by a discharge state where ions from the higher concentration solution reincorporate into their ion selective electrode . This charge/discharge cycle produces extractable energy per cycle as described in the figure shown.
In the present work, the same group investigates replacing the cathode material with a higher capacity material, Na4Mn9O18, as opposed to the previously reported Na2M5O10. An overall improvement was observed when researchers simulated batteries hooked up in a series, by passing the same effluent wastewater 12 times through the same cell. In doing so, the cumulative energy produced was 0.44 kWh/m3 of wastewater, compared to the theoretical maximum of 0.65 kWh/m3. This denotes an overall efficiency of 68%. Future considerations include reducing the number of batteries required in series, as well as eliminating the use of silver as a Cl– selective electrode, for environmental concerns.
1.) Nano Lett., 2011, 11, 1810–1813
Interested? Read the full advance article in Energy and Environmental Science here:
Meng Ye, Mauro Pasta, Xing Xie, Yi Cui, and Craig S. Criddle
Energy Environ. Sci., 2014, Advance Article
DOI: 10.1039/C4EE01034E