Top 25 most-read Energy & Environmental Science articles for Q1
Review of solutions to global warming, air pollution, and energy security
Mark Z. Jacobson
DOI: 10.1039/B809990C
Graphene based new energy materials
Yiqing Sun, Qiong Wu and Gaoquan Shi
DOI: 10.1039/C0EE00683A
Organic tandem solar cells: A review
Tayebeh Ameri, Gilles Dennler, Christoph Lungenschmied and Christoph J. Brabec
DOI: 10.1039/B817952B
Bulk nanostructured thermoelectric materials: current research and future prospects
A. J. Minnich, M. S. Dresselhaus, Z. F. Ren and G. Chen
DOI: 10.1039/B822664B
Flexible energy storage devices based on graphene paper
Hyeokjo Gwon, Hyun-Suk Kim, Kye Ung Lee, Dong-Hwa Seo, Yun Chang Park, Yun-Sung Lee, Byung Tae Ahn and Kisuk Kang
DOI: 10.1039/C0EE00640H
Carbon nanotubes for lithium ion batteries
Brian J. Landi, Matthew J. Ganter, Cory D. Cress, Roberta A. DiLeo and Ryne P. Raffaelle
DOI: 10.1039/B904116H
Development and challenges of LiFePO4 cathode material for lithium-ion batteries
Li-Xia Yuan, Zhao-Hui Wang, Wu-Xing Zhang, Xian-Luo Hu, Ji-Tao Chen, Yun-Hui Huang and John B. Goodenough
DOI: 10.1039/C0EE00029A
Graphene-based nanomaterials for energy storage
Martin Pumera
DOI: 10.1039/C0EE00295J
Highly active cobalt phosphate and borate based oxygen evolving catalysts operating in neutral and natural waters
Arthur J. Esswein, Yogesh Surendranath, Steven Y. Reece and Daniel G. Nocera
DOI: 10.1039/C0EE00518E
Nanostructured silicon for high capacity lithium battery anodes
Jeannine R. Szczech and Song Jin
DOI: 10.1039/C0EE00281J
Development of alternative photocatalysts to TiO2: Challenges and opportunities
María D. Hernández-Alonso, Fernando Fresno, Silvia Suárez and Juan M. Coronado
DOI: 10.1039/B907933E
CO2 capture by solid adsorbents and their applications: current status and new trends
Qiang Wang, Jizhong Luo, Ziyi Zhong and Armando Borgna
DOI: 10.1039/C0EE00064G
Vertically-aligned nanostructures of ZnO for excitonic solar cells: a review
Irene Gonzalez-Valls and Monica Lira-Cantu
DOI: 10.1039/B811536B
Carbon nanostructures for energy
Nazario Martin, Dirk M. Guldi, Andreas Hirsch
DOI: 10.1039/C1EE90001C
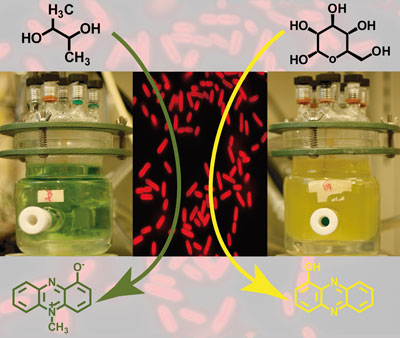
Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels
Juan Carlos Serrano-Ruiz and James A. Dumesic
DOI: 10.1039/C0EE00436G
Green energy storage materials: Nanostructured TiO2 and Sn-based anodes for lithium-ion batteries
Da Deng, Min Gyu Kim, Jim Yang Lee and Jaephil Cho
DOI: 10.1039/B823474D
The role of buffer layers in polymer solar cells
Riccardo Po, Chiara Carbonera, Andrea Bernardi and Nadia Camaioni
DOI: 10.1039/C0EE00273A
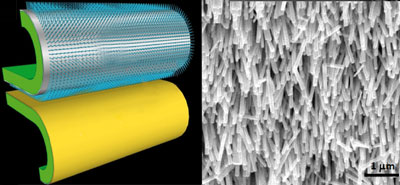
Electrospun nanofibers in energy and environmental applications
V. Thavasi, G. Singh and S. Ramakrishna
DOI: 10.1039/B809074M
Olivine LiFePO4: development and future
Yonggang Wang, Ping He and Haoshen Zhou
DOI: 10.1039/C0EE00176G
Nanostructured carbon-based electrodes: bridging the gap between thin-film lithium-ion batteries and electrochemical capacitors
Seung Woo Lee, Betar M. Gallant, Hye Ryung Byon, Paula T. Hammond and Yang Shao-Horn
DOI: 10.1039/C0EE00642D
Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics
Peter J. le B. Williams and Lieve M. L. Laurens
DOI: 10.1039/B924978H
High-performance Si microwire photovoltaics
Michael D. Kelzenberg, Daniel B. Turner-Evans, Morgan C. Putnam, Shannon W. Boettcher, Ryan M. Briggs, Jae Yeon Baek, Nathan S. Lewis and Harry A. Atwater
DOI: 10.1039/C0EE00549E
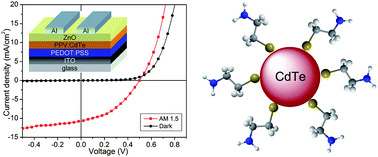
Bulk-heterojunction hybrid solar cells based on colloidal nanocrystals and conjugated polymers
Yunfei Zhou, Michael Eck and Michael Krüger
DOI: 10.1039/C0EE00143K
An overview of CO2 capture technologies
Niall MacDowell, Nick Florin, Antoine Buchard, Jason Hallett, Amparo Galindo, George Jackson, Claire S. Adjiman, Charlotte K. Williams, Nilay Shah and Paul Fennell
DOI: 10.1039/C004106H
Design of solid catalysts for the conversion of biomass
Roberto Rinaldi and Ferdi Schüth
DOI: 10.1039/B902668A












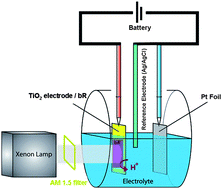 It is thought the proton pumping property of bR can be used in a variety of applications, especially those related to third generation photovoltaic cells.
It is thought the proton pumping property of bR can be used in a variety of applications, especially those related to third generation photovoltaic cells.