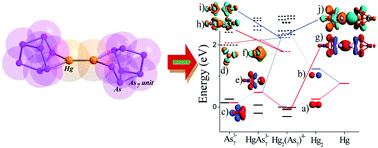
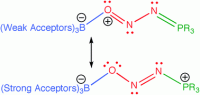
 Thomas Gilbert from Northern Illinois University reports on a computational study looking at Ar3B–ONN–PR3 complexes that result from reactions between borane-phosphine frustrated Lewis pairs and N2O. By exploring the structure, stability, mechanism of formation and functionalisation of Ar3B–ONN–PR3 complexes, we can further understand how frustrated Lewis pairs can capture small molecules, like N2O, and hold them for derivatisation, in a similar way to enzymes.
Thomas Gilbert from Northern Illinois University reports on a computational study looking at Ar3B–ONN–PR3 complexes that result from reactions between borane-phosphine frustrated Lewis pairs and N2O. By exploring the structure, stability, mechanism of formation and functionalisation of Ar3B–ONN–PR3 complexes, we can further understand how frustrated Lewis pairs can capture small molecules, like N2O, and hold them for derivatisation, in a similar way to enzymes.
A key finding from this study is a symbiotic relationship that forms between Lewis acid and Lewis base to overcome endothermic or weakly exothermic energetics, forming one structural isomer in preference to others. Gilbert makes several other exciting conclusions so download the manuscript whilst it’s free to find out more….
Computational studies of complexation of nitrous oxide by borane–phosphine frustrated Lewis pairs
Thomas M. Gilbert
Dalton Trans., 2012
DOI: 10.1039/C2DT30208J
Frustrated Lewis pairs are compounds or mixtures combining sterically hindered Lewis donors and acceptors. Such systems are capable of unique reactivity with fascinating applications in stoichiometry and catalysis. Dalton Transactions will be publishing a themed issue on frustrated Lewis pairs later in the year – below are some other articles due to be included in the issue:
Exchange chemistry of tBu3P(CO2)B(C6F5)2Cl
Rebecca C. Neu, Gabriel Ménard and Douglas W. Stephan
Dalton Trans., 2012
DOI: 10.1039/C2DT30206C
Dimeric aluminum–phosphorus compounds as masked frustrated Lewis pairs for small molecule activation
Steffi Roters, Christian Appelt, Hauke Westenberg, Alexander Hepp, J. Chris Slootweg, Koop Lammertsma and Werner Uhl
Dalton Trans., 2012
DOI: 10.1039/C2DT30080J











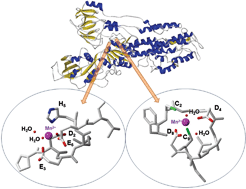 In this HOT article, Zoroddu and coworkers present NMR (mono- and bi-dimensional) and EPR analysis of Zn and Mn complexation to small fragments of the Park9 gene (a member of the P5-type ATPase family) important in
In this HOT article, Zoroddu and coworkers present NMR (mono- and bi-dimensional) and EPR analysis of Zn and Mn complexation to small fragments of the Park9 gene (a member of the P5-type ATPase family) important in 
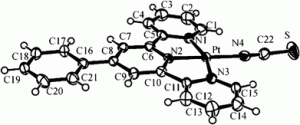
![solvent accessible voids in [Pt{4'-(Ph)trpy}(NCS)]SbF6 solvent accessible voids in [Pt{4'-(Ph)trpy}(NCS)]SbF6](https://blogs.rsc.org/dt/files/2012/03/Pt-terpyridine-complex-solvent-300x145.gif)