|
Even 70 years after its discovery, the behaviour of plutonium still remains something of an enigma. Now Swedish researchers have discovered that plutonium colloids shrink with age becoming very small (3–4 atoms) with a shortening of the average Pu–O bond and Pu⋯Pu distances, consistent with partial oxidation having occurred. Two types of colloidal materials were studied: high-fired crystalline PuO2, and freshly prepared Pu(IV) colloids. The structures of both types were determined by EXAFS at the beginning of the experiment and after controlled aging of up to five years. The results show that even freshly prepared Pu(IV) colloids are polynuclear nano clusters with PuO2 structure, bringing new insight into the evolution of plutonium with time. |
The structure of plutonium(IV) oxide as hydrolysed clusters in aqueous suspensions
Christian Ekberg, Kristian Larsson, Gunnar Skarnemark, Arvid Ödegaard-Jensen and Ingmar Persson
Dalton Trans., 2013, Advance Article
DOI: 10.1039/C2DT32185H
Other Dalton papers by the same author are:
Crystal structure of [Eu(CyMe4-BTBP)2κ2O,O‘-(NO3)](NO3)2·n-C8H17OH and its structure in 1-octanol solution
Daniel Lundberg, Ingmar Persson and Christian Ekberg
Dalton Trans., 2013, Advance Article
DOI: 10.1039/C2DT32317F, Communication
Radiolysis of solvents containing C5-BTBP: identification of degradation products and their dependence on absorbed dose and dose rate
Anna Fermvik, Laurence Berthon, Christian Ekberg, Sofie Englund, Teodora Retegan and Nicole Zorz
Dalton Trans., 2009, 6421-6430
DOI: 10.1039/B907084B, Paper












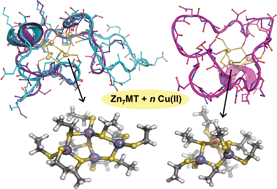
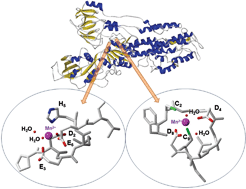 In this HOT article, Zoroddu and coworkers present NMR (mono- and bi-dimensional) and EPR analysis of Zn and Mn complexation to small fragments of the Park9 gene (a member of the P5-type ATPase family) important in
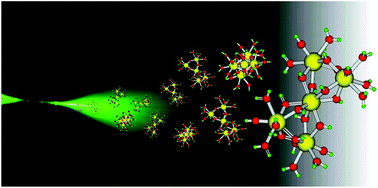
In this HOT article, Zoroddu and coworkers present NMR (mono- and bi-dimensional) and EPR analysis of Zn and Mn complexation to small fragments of the Park9 gene (a member of the P5-type ATPase family) important in 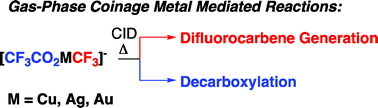 The presence of C–F bonds in organic compounds has a dramatic influence on their physical, chemical and biological properties. Metal mediated reactions that result in C–F bond formation or C–F bond activation have attracted considerable attention as the demand for organofluorine compounds has increased. Metal-catalysed decarboxylative cross-coupling reactions using trifluoracetate as a cheap source of introducing “CF3” represents an attractive approach. Until now, little has been known about the fragmentation mechanisms of metal trifluoracetates. In this Dalton Transactions HOT article, Rijs and O’Hair use a combination of gas-phase 3D quadrupole ion trap mass spectrometry experiments and density functional theory (DFT) calculations to examine the mechanism of thermal decomposition of fluorinated coinage metal carboxylates. Synthetic chemists should be able to use these results to design new ways of incorporating CF3 and F fragments using trifluoroacetate.
The presence of C–F bonds in organic compounds has a dramatic influence on their physical, chemical and biological properties. Metal mediated reactions that result in C–F bond formation or C–F bond activation have attracted considerable attention as the demand for organofluorine compounds has increased. Metal-catalysed decarboxylative cross-coupling reactions using trifluoracetate as a cheap source of introducing “CF3” represents an attractive approach. Until now, little has been known about the fragmentation mechanisms of metal trifluoracetates. In this Dalton Transactions HOT article, Rijs and O’Hair use a combination of gas-phase 3D quadrupole ion trap mass spectrometry experiments and density functional theory (DFT) calculations to examine the mechanism of thermal decomposition of fluorinated coinage metal carboxylates. Synthetic chemists should be able to use these results to design new ways of incorporating CF3 and F fragments using trifluoroacetate.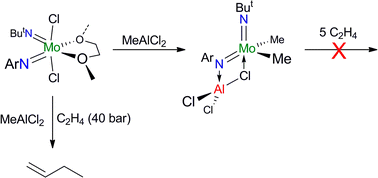 Despite the fact that the catalytic activity of Cr and W imido complexes in ethylene dimerization processes has been studied by various authors, to date the analogous Mo catalysts have not been studied. In this HOT article, Dyer et al. set out to fill this gap in our knowledge and several molybdenum bis(imido) complexes were tested for ethylene dimerization catalysis in combination with EtAlCl2, showing moderate activity when bulky aryl substituents at the imido ligand are employed. In contrast, when MeAlCl2 is used the activity of the catalyst decreases considerably. To understand the role of the activator in these processes the authors have determined the molecular structures of several complexes derived of the reaction of bis(imido) molybdenum compounds with different aluminium halide reagents.
Despite the fact that the catalytic activity of Cr and W imido complexes in ethylene dimerization processes has been studied by various authors, to date the analogous Mo catalysts have not been studied. In this HOT article, Dyer et al. set out to fill this gap in our knowledge and several molybdenum bis(imido) complexes were tested for ethylene dimerization catalysis in combination with EtAlCl2, showing moderate activity when bulky aryl substituents at the imido ligand are employed. In contrast, when MeAlCl2 is used the activity of the catalyst decreases considerably. To understand the role of the activator in these processes the authors have determined the molecular structures of several complexes derived of the reaction of bis(imido) molybdenum compounds with different aluminium halide reagents.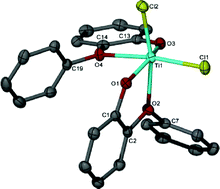 In this HOT article, several new Ti(IV) complexes bearing aryloxy or alkoxy ligands have been prepared and evaluated as catalysts for the oligomerization/polymerization of ethylene. Obviously, polyethylene is the major product but ethylene oligomers also result, ranging from dimers to higher oligomers. The results indicate a number of different active species are formed upon activation, with oligomers likely arising through a metallacyclic mechanism.
In this HOT article, several new Ti(IV) complexes bearing aryloxy or alkoxy ligands have been prepared and evaluated as catalysts for the oligomerization/polymerization of ethylene. Obviously, polyethylene is the major product but ethylene oligomers also result, ranging from dimers to higher oligomers. The results indicate a number of different active species are formed upon activation, with oligomers likely arising through a metallacyclic mechanism.