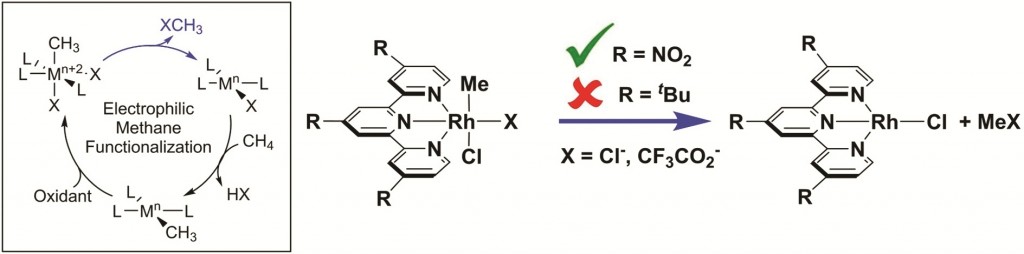
In their recent paper in Dalton Transactions, Stasch, Jones and co-workers describe the use of bulky ring-expanded N-heterocyclic carbenes (NHCs) for the stabilisation of Group 15 trichlorides, ECl3; E = P, As, Sb.
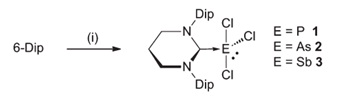
The authors show that mixing 1:1 solutions of the ring-expanded, 2,6-diisopropylphenyl-subsitutied NHC “6-Dipp” with ECl3 (E = P, As, and Sb) affords satisfactory yields of the corresponding [(6-Dipp)ECl3] adduct, noting that the use of the alternatively-substituted mesityl-substituted carbene (6-Mes) led to a mixture of products thus highlighting the importance of the 6-Dipp ligand for stabilising the adducts.
The group characterised each adduct using standard means, including NMR spectroscopy and electron-impact (EI) mass spectrometry. In the cases of P and Sb, they were able to use X-ray crystallography to determine the solid state structures of those compounds and observed that each pnictogen centre adopted a saw-horse geometry.
When the researchers tried to reduce these adducts using either KC8 or a Mg(I) derivative, they were mostly unsuccessful, however they were able to reduce [(6-Dipp)PCl3] using the former reagent to form a unique dicationic carbene-stabilized P4 unit. Once again, the authors attributed the stability of this species to the steric profile of the 6-Dipp ligand framework. The X-ray crystal structure of the complex shows a P4-butterfly geometry stabilised by two carbene moieties.
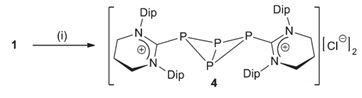
During the course of their reactivity studies, the group identified (6-MesH)2 as a byproduct resulting from reaction of in-situ generated [(6-Mes)PCl3] with KC8. With further optimisation, they revealed that this moiety could be accessed from treatment of [6-MesH]Br with KC8 – marking the first successful reductive coupling of cyclic amidinium ions. Along with NMR and X-ray crystal data, a cyclic voltammetry (CV) study was also performed on (6-MesH)2 to fully characterise this unique species.
The successful syntheses of all these p-block NHC complexes pave the way for new discoveries in fundamental reactivity, bonding, and catalysis employing main group elements, further demonstrating the potential of these elements to perform exciting chemistry.
Read the full article to find out more:
Expanded Ring N-Heterocyclic Carbene Adducts of Group 15 Element Trichlorides: Synthesis and Reduction studies
Anastas Sidiropoulos, Brooke Osborne, Alexandr Simonov, Deepak Dange, Alan Bond, Andreas Stasch and Cameron Jones
Dalton Trans., 2014, DOI: 10.1039/C4DT02074J
 |
Marcus Drover is a Ph.D. student, co-supervised by Professors Laurel Schafer and Jennifer Love at the University of British Columbia. His research is focused on the preparation of low-coordinate RhI and IrI complexes for use in small-molecule reactivity. He grew up in St. John’s, Newfoundland and graduated from Memorial University (MUN) before beginning graduate school in 2012. |















 We are delighted to announce four new
We are delighted to announce four new