Posted on behalf of Ian Mallov, web writer for Dalton Transactions
Reading the chemical literature as a synthetic chemist, I can often empathize with the story of the practical challenges which underlies the research story in a paper. This article from Gunnoe and co-workers certainly left me with an appreciation for the gutsy way in which the researchers overcame significant challenges to the usual synthetic and analytical techniques of the inorganic chemist to present some hard-won gains on the reductive elimination chemistry of rhodium.
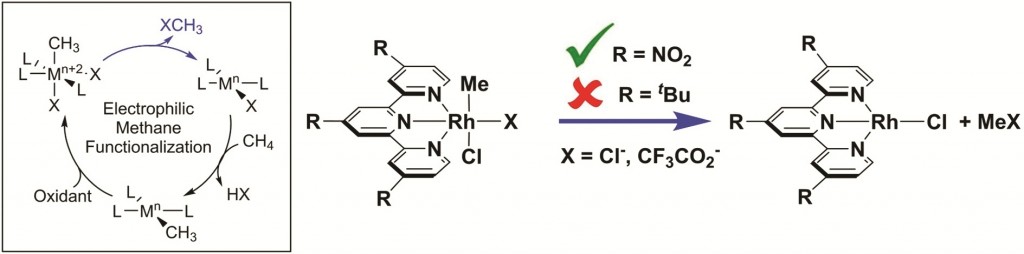
The power of reductive elimination – inducing two chemical moieties bonded to a central atom to leave without one of their electrons, resulting in a formal gain of electrons by this central atom – lies in its ability to fuse together the two groups leaving. In this way chemical bonds which are difficult or impossible to form by other means can be created. When the elimination of one group breaks a metal-carbon bond, the possibility to form a carbon-X bond with the other group reductively-eliminated, and thus functionalize a carbon centre, is particularly attractive.
Such is the technique the Gunnoe group present here. Much more commonly used in platinum chemistry, they prove this approach to be applicable to rhodium chemistry also, inducing reductive elimination of CH3 and a range of halides or pseudo-halides. While the CH3-X products formed are useful only as a proof of principle, the proof was a result of overcoming significant challenges.
The Rh-terpyridine complex ultimately coaxed to undergo reductive CH3-X elimination was so insoluble that they were unable to obtain a NMR spectrum, much less an x-ray crystal structure, and had to trust that a combustion analysis supporting their hypothesized product was evidence enough to proceed. Then, the hoped-for reductive elimination did not occur until electron-withdrawing NO2 groups were installed on the terpyridine backbone, the reaction was heated, and the solvent changed to CD3NO2 (certainly not the first solvent one would have tried). Moreover, the CH3-X products formed were gases, making it very difficult to quantify the amount produced.
Nonetheless, their persistent tweaks of the organometallic complex itself, aided by computational thermodynamic data and the use of second-choice analytical techniques when necessary yielded insight into Rh reductive elimination.
Find out more and download the article now:
Reductive functionalization of a rhodium(III)–methyl bond by electronic modification of the supporting ligand
M. E. O’Reilly, D. R. Pahls, J. R. Webb, N. C. Boaz, S. Majumdar, C. D. Hoff, J. T. Groves, T. R. Cundari and T. B. Gunnoe
Dalton Trans., 2014, DOI: 10.1039/C4DT00234B
 |
Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school. |