Direct capture of CO2 from the air presents significant challenges, one of which is the separation of CO2 from other gases. Currently, a lot of waste carbon dioxide is captured at the emission source – fossil fuel-powered energy plants – by a ‘filter’ that traps the CO2 as it travels up a chimney. This method can prevent up to 90% of a power plant’s carbon emissions from entering the atmosphere, however the process requires a lot of energy and the captured gas still needs to be transported to a suitable storage area. New technologies for selective capture of carbon dioxide from the air are still in their infancy, but could offer more efficient ways to trap CO2 anywhere on the planet, not just at the sources of emission.
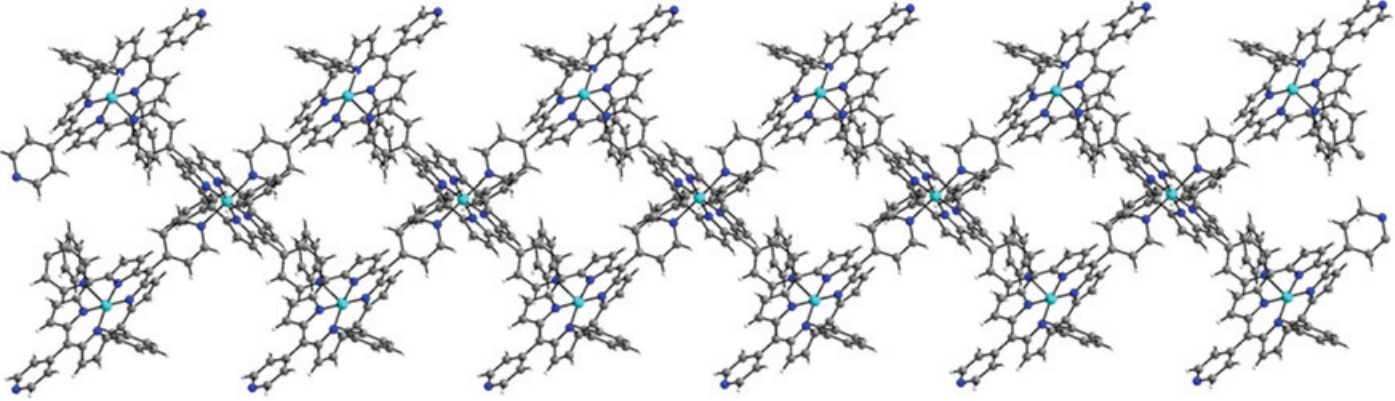
Figure 1: Double chain MOF
Metal organic frameworks (MOFs), also known as coordination polymers, are compounds containing metal ions which are coordinated to organic molecules to form extensive two- or three-dimensional structures. The uniform and controllable porosity of these materials has already been exploited as potential devices for gas capture and storage.2 In one recent paper in Dalton Transactions, Kim and co-workers synthesise novel MOFs using cobalt and zinc metal ions coordinated to porphyrin-based molecules. The resulting MOFs display an interesting 1D ‘double chain’ arrangement of molecules (Figure 1). These ‘double chains’ pack together tightly, forming hydrogen bonds between the ‘chains’, resulting in a stable solid state structure with defined pores. Gas sorption experiments revealed that these new MOFs both show high uptake of CO2 gas compared with nitrogen (N2), hydrogen (H2) and methane (CH4), (Figure 2), making these types of materials excellent candidates for selective CO2 capture from the air.
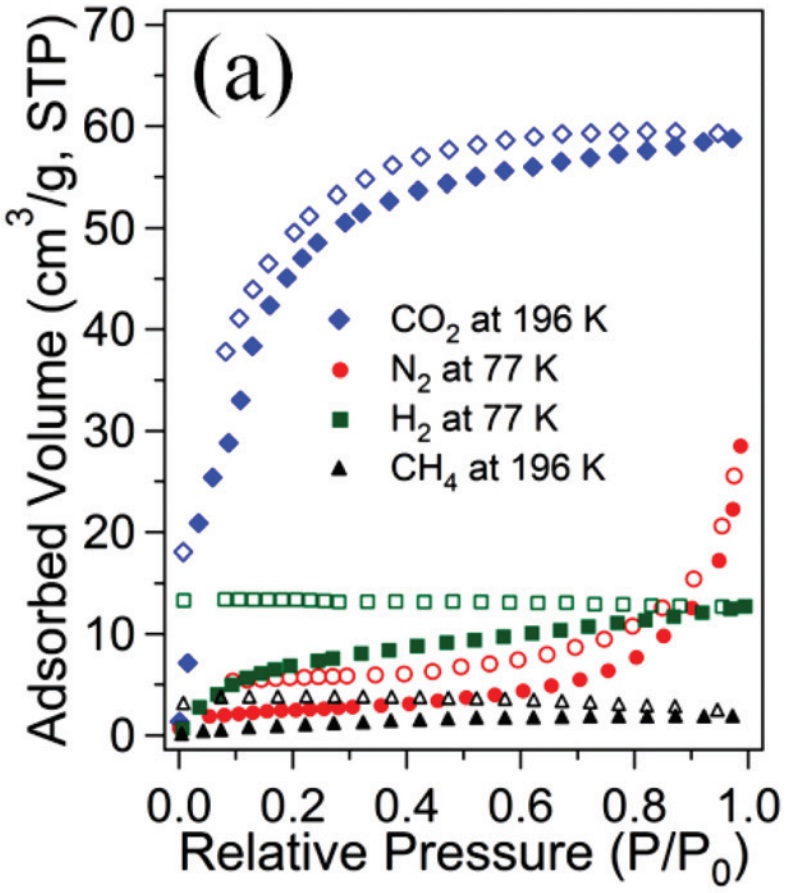
Figure 2: Gas uptake by double-chain MOF
Find out more and download the article now:
CO2 selective 1D double chain dipyridyl-porphyrin based porous coordination polymers
Dalton Trans. 2014, DOI:10.1039/C3DT53287A
References
1 IPCC Special Report, ‘Carbon Dioxide Capture and Storage’, IPCC Working Group III, 2005.
2 S. L. James, Chem. Soc. Rev., 2003, 32, 276.
 |
Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds. |










