Posted on behalf of Liana Allen, web writer for Dalton Transactions
As more information becomes known about the negative impacts that humans are having on the Earth’s environment, increasing focus is being put on ways of decreasing these effects in all aspects of our lives. For the chemical industry, there are already several key areas which have been identified as needing particular attention with respect to decreasing our effect on the environment. In the last decade, this challenge has become so important that it is now recognized as a field in its own right, referred to as ‘Green Chemistry’. Some of the overall themes of green chemistry are: decrease of the amount of waste by-products generated by a reaction (i.e. avoiding poor atom economy reagents), reduction of reagents which pose safety hazards (i.e. explosives and known toxic compounds), and ‘cleaner’ solvent choices (i.e. avoiding chlorinated solvents, or volatile solvents whose vapours pose an environmental problem).1
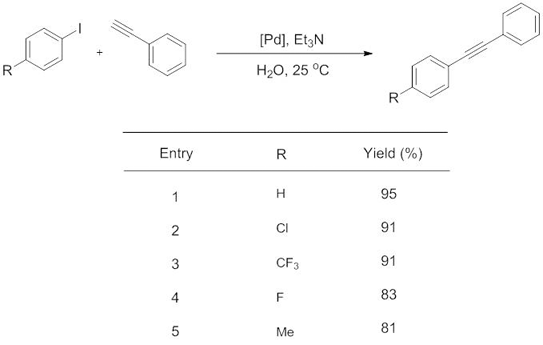
One of the most important reactions used in chemical industry is formation of carbon-carbon bonds.2 The methodology most commonly used to form such bonds is a group of reactions called palladium-catalysed cross-couplings. One of this set of vital reactions is called the Sonogashira coupling. This reaction is used to couple terminal alkynes with aryl or vinyl halides to form di-substituted alkynes, and requires a copper co-catalyst (in addition to a palladium catalyst), heating conditions, and a solvent such as DMF.
In their recent Dalton Transactions article, Wang and colleagues take significant steps towards applying the principles of green chemistry to the Sonogashira reaction by optimizing new conditions where; (a) a copper co-catalyst is not required, thus increasing the atom efficiency of the reaction, (b) the reaction can be run at room temperature, eradicating the energy cost normally required for heating and (c) the solvent is readily available and environmentally benign water.
To read more, see:
Copper-free Sonogashira Cross-Coupling Reaction Promoted by Palladium complexes of Nitrogen-containing Chelating Ligand in Neat Water at Room Temperature
Hong Zhong, Jinyun Wang, Liuyi Li and Ruiha Wang, Dalton Trans. 2013, DOI:10.1039/C3DT52970C
1 Constable, D. J. C. et al., Green Chem., 2007, 9, 411.
2 Carey, J. S. et al., Org. Biomol. Chem., 2006, 4, 2337.
 Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.
Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.