Now welcoming abstract submissions for Dalton Discussion 14 – Advancing the Chemistry of the f-Elements
28th – 30th July 2014, Edinburgh, UK
| Confirmed speakers: | Geoff Cloke, University of Sussex, UK |
| Melissa Denecke, Karlsruhe Institute of Technology, Germany | |
| Laurent Maron, Université Paul Sabatier, Toulouse, France | |
| Jeffrey Long, University of California, Berkeley, USA | |
| Nik Kaltsoyannis, University College London, UK | |
| Marinella Mazzanti, CEA, Grenoble, France | |
| Paula Diaconescu, University of California, Los Angeles, USA | |
| Jonathan Lloyd, University of Manchester, UK |
This Dalton Discussion aims to highlight the burgeoning role, and exciting prospects for f-elements in modern, metal-based chemistry. Abstracts for oral presentations must be sent in before 16th December 2013.
Authors of the selected oral abstracts will then be expected to submit their work as a full paper, which will form the basis of their short presentation at the Discussion meeting. The paper itself must contain new, unpublished work and be submitted for review to the Editorial Office of Dalton Transactions by 30th June 2014. At the conference, each author will be given approximately 5 minutes to summarise the key points of their paper in order to leave the majority of time open for discussion
This year, Dalton Discussion 14 is adopting a new process. Papers presented at Dalton Discussions will continue to be published in a dedicated themed issue of Dalton Transactions, however article peer-review will now take place after the Discussion. This means that speakers have longer to make the finishing touches to their article before the papers are made available online prior to the meeting.
To find out more about Dalton Discussion 14 and to submit your abstract, visit the dedicated webpage.












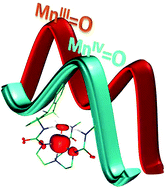
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/h2_char_e001.gif) O complexes: how important is the oxo ligand basicity in the C–H activation step?
O complexes: how important is the oxo ligand basicity in the C–H activation step?