These are the most accessed Dalton Transactions articles during the last 3 months (July to Septmeber):
Recent progress in the synthesis of inorganic nanoparticles
C. N. R. Rao, H. S. S. Ramakrishna Matte, Rakesh Voggu and A. Govindaraj
Dalton Trans., 2012,41, 5089-5120
DOI: 10.1039/c2dt12266a
Asymmetric cyclopropanation of olefins catalysed by Cu(I) complexes of chiral pyridine-containing macrocyclic ligands (Pc-L*)
Brunilde Castano, Stefano Guidone, Emma Gallo, Fabio Ragaini, Nicola Casati, Piero Macchi, Massimo Sisti and Alessandro Caselli
Dalton Trans., 2013,42, 2451-2462
DOI: 10.1039/c2dt32347h
C–H functionalization: thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity
Georgiy B. Shul’pin
Dalton Trans., 2013,42, 12794-12818
DOI: 10.1039/c3dt51004b
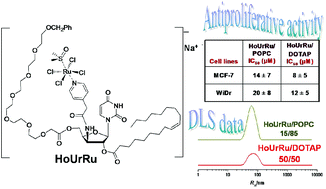
Ferrocene-based compartmental ligand for the assembly of neutral ZnII/LnIII heterometallic complexes
Vadapalli Chandrasekhar, Amit Chakraborty and E. Carolina Sañudo
Dalton Trans., 2013,42, 13436-13443
DOI: 10.1039/c3dt51432c
A fluorescence ‘turn-on’ chemodosimeter for selective detection of Nb5+ ions in mixed aqueous media
Abhishek Kumar Gupta, Abhimanew Dhir and Chullikkattil P. Pradeep
Dalton Trans., 2013,42, 12819-12823
DOI: 10.1039/c3dt50914a
Phosphonate coupling molecules for the control of surface/interface properties and the synthesis of nanomaterials
Gilles Guerrero, Johan G. Alauzun, Michel Granier, Danielle Laurencin and P. Hubert Mutin
Dalton Trans., 2013,42, 12569-12585
DOI: 10.1039/c3dt51193f
A new fluorescent probe for distinguishing Zn2+ and Cd2+ with high sensitivity and selectivity
Yiqun Tan, Junkuo Gao, Jiancan Yu, Ziqi Wang, Yuanjing Cui, Yu Yang and Guodong Qian
Dalton Trans., 2013,42, 11465-11470
DOI: 10.1039/c3dt50991e
Multifunctional NH2-mediated zirconium metal–organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr(VI)
Lijuan Shen, Shijing Liang, Weiming Wu, Ruowen Liang and Ling Wu
Dalton Trans., 2013,42, 13649-13657
DOI: 10.1039/c3dt51479j
Approaches to efficient molecular catalyst systems for photochemical H2 production using [FeFe]-hydrogenase active site mimics
Mei Wang, Lin Chen, Xueqiang Li and Licheng Sun
Dalton Trans., 2011,40, 12793-12800
DOI: 10.1039/c1dt11166c
Do you have any comments or thoughts on these articles? Why not leave these in the comment box below.
If you have an article to submit to Dalton Transactions, please submit to us here!