With current oil consumption levels high, light crude oil reserves will be exhausted, giving way to heavy and extra heavy bitumen refinery feeds. These contain a higher proportion of large polycyclic hydrocarbons, together with N- and S-heteroaromatics, which need to be removed or transformed, without catalyst poisoning. Current methods to do this require high temperatures and pressures.
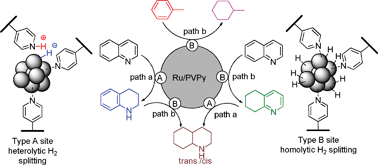
The catalyst made by the researchers, composed of ruthenium nanoparticles immobilised on a polymer, hydrogenates aromatic compounds under moderate conditions with no poisoning.
Hydrogenation of arenes and N-heteroaromatic compounds over ruthenium nanoparticles on poly(4-vinylpyridine): a versatile catalyst operating by a substrate-dependent dual site mechanism
Minfeng Fang, Nataliya Machalaba and Roberto A. Sánchez-Delgado
Dalton Trans., 2011, DOI: 10.1039/C1DT10801H










