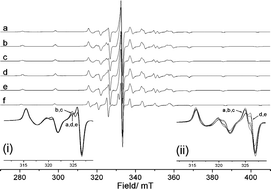
 In this HOT article, the authors have extended their work in understanding enantioselective catalysis by coordination compounds. The role of steric hindrance in controlling the binding mode of propylene oxide to a novel vanadyl salen-type complex N,N‘-bis(5-tert-butylsalicylidene)-1,2-cyclohexanediamino-vanadium(IV) oxide, [VO(3)], has been investigated using CW/pulse EPR, ENDOR and HYSCORE spectroscopy and compared to that of the parent complex N,N‘-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamino-vanadium(IV) oxide, [VO(1)]. Removal of the ‘inner tert-butyl groups from the salicylidene rings reduces the steric hindrance between the ligand and epoxide substrate. As a result the selectivity for binding single enantiomers of propylene oxide in these complexes is reversed in [VO(3)] relative to [VO(1)].
In this HOT article, the authors have extended their work in understanding enantioselective catalysis by coordination compounds. The role of steric hindrance in controlling the binding mode of propylene oxide to a novel vanadyl salen-type complex N,N‘-bis(5-tert-butylsalicylidene)-1,2-cyclohexanediamino-vanadium(IV) oxide, [VO(3)], has been investigated using CW/pulse EPR, ENDOR and HYSCORE spectroscopy and compared to that of the parent complex N,N‘-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamino-vanadium(IV) oxide, [VO(1)]. Removal of the ‘inner tert-butyl groups from the salicylidene rings reduces the steric hindrance between the ligand and epoxide substrate. As a result the selectivity for binding single enantiomers of propylene oxide in these complexes is reversed in [VO(3)] relative to [VO(1)].
Read more for FREE at:
Structure and pulsed EPR characterization of N,N‘-bis(5-tert-butylsalicylidene)-1,2-cyclohexanediamino-vanadium(IV) oxide and its adducts with propylene oxide
E. Carter, I. A. Fallis, B. M. Kariuki, I. R. Morgan, D. M. Murphy, T. Tatchell, S. Van Doorslaer and E. Vinck
Dalton Trans., 2011, Advance Article
DOI: 10.1039/C1DT10378D











