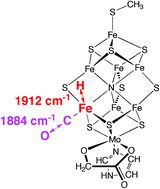
Fe–H stretching frequencies occur in the same spectral window as the C–O stretching frequencies, with lesser intensity, and both stretches are strongly coupled in some structures. Symmetrical bridging of CO between two Fe atoms of FeMo-co is destabilised by the presence of other ligands on Fe, and the reason for this is evident. Two results for bound formyl, HCO, are reported. These calculations of reference structures allow some interpretation of existing experimental spectra, but, perhaps more significantly, they suggest further kinetic infrared experiments to elucidate the chemical mechanism of catalysis by nitrogenase under normal turnover conditions.
Read more about nitrogenase mechanisms for FREE until 22nd June at:
Calculated vibrational frequencies for FeMo-co, the active site of nitrogenase, bearing hydrogen atoms and carbon monoxide
Ian Dance
Dalton Trans., 2011, Advance Article
DOI: 10.1039/C1DT10505