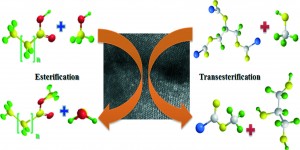
Biodiesel can be derived from oil found in animal and plant matter or even from recycled cooking oil. Much research has investigated the catalysis of the esterification and transesterification reactions needed to synthesize biodiesel. In this advance article, Patel and Narkhede contribute a novel catalyst towards the advancement of biodiesel as a competitive renewable energy source.
The authors used mono-lacunary silicotungstate supported on MCM-41, a mobile crystalline material hexagonal silicate, for the acid catalyzed esterification of oleic acid. They optimized the reaction up to 81% conversion with elevated temperature and prolonged reaction times. The researchers demonstrated the heterogenous catalyst could be reused in four iterative reactions without any appreciable loss in activity.
The transesterification of other oils including waste cooking oil, jatropha oil, sunflower oil, cotton seed oil and mustard oil was catalyzed in excellent yields. This catalyst is promising as an environmentally benign component of biodiesel production, though more research needs to be done to decrease the high catalyst loadings.
Click the link below to read more:
Biodiesel synthesis via esterification and transesterification over a new heterogeneous catalyst comprising lacunary silicotungstate and MCM-41
Anjali Patel and Nilesh Narkhede
 Tien Nguyen is working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis.
Tien Nguyen is working towards her PhD in David Nicewicz’s research group at the University of North Carolina at Chapel Hill, USA. Her current area of research focuses on anti-Markovnikov hydroamination of alkenes using photoredox catalysis.