The topic of sustainable energy needs no introduction and while there are numerous technologies that provide a more sustainable and environmentally friendly energy source, no single process has been identified as the best way forward. This is in part due to the fact that current supplies of energy, whether generated by gas, coal, nuclear, solar, wind or tidal sources, all have their own disadvantages.
Biodiesel is no different, as the transesterification of readily available vegetable oils produces large quantities of waste glycerol as a by-product (around 10 wt%) causing substantial economic waste.
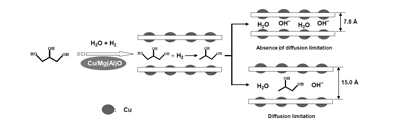
In their manuscript Binbin Zhao, Chengcheng Li and Chunli Xu discuss their attempts to understand the conversion of glycerol into the more useful product 1,2-propanediol. The reaction mechanism of glycerol hydrogenolysis is not well understood so the team have tried to study it using a Cu/Mg-Al mixed-oxide catalyst with hydrotalcite-like compounds (a class of anionic clays found in nature).
You can download the manuscript below for full details of the team’s research
Insight of catalytic mechanism of glycerol hydrogenolysis using basal spacing of hydrotalcite as a tool
Binbin Zhao, Chengcheng Li and Chunli Xu
Journal Article
Catal. Sci. Technol., 2012, Accepted Manuscript
DOI: 10.1039/C2CY20144E
If you’re interested in finding out more information about the role of catalysis in sustainable energy you can take a look at our previous blog post The quest for cleaner, cheaper, more sustainable energy.
Of course you can keep up to date with the latest news in catalysis hassle free, by following us on twitter, liking us on facebook, or signing up to our e-alerts!