Read more for FREE at:
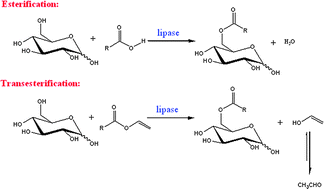
Enzymatic synthesis of sugar fatty acid esters in ionic liquids
Zhen Yang and Ze-Lin Huang
Catal. Sci. Technol., 2012, Advance Article
DOI: 10.1039/C2CY20109G

Read more for FREE at:
Enzymatic synthesis of sugar fatty acid esters in ionic liquids
Zhen Yang and Ze-Lin Huang
Catal. Sci. Technol., 2012, Advance Article
DOI: 10.1039/C2CY20109G
Scientists from Japan have used waste slag (the high volume byproduct from iron making) to catalyse biofuel synthesis. Not only does this engineering strategy find a much-needed recycling alternative to slag, but renewable fuel, biodiesel is produced – a sustainable and environmentally-friendly alternative to petrochemicals.
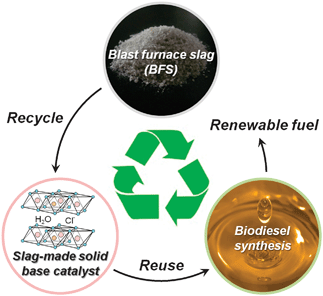
Use of the slag-made hydrocalumite catalysts for large scale biodiesel synthesis promises an economical and ecological contribution to alleviate the fuel demands of the future.
Read the article here: Transesterifications Using Hydrocalumite Synthesized from Waste Slag: An Economical and Ecological Route for Biofuel Production
Yasutaka Kuwahara, Keita Tsuji, Tetsutaro Ohmichi, Takashi Kamegawa, Kohsuke Mori and Hiromi Yamashita
Want to find out what is being published in catalysis research? The Catalysis Science & Technology e-alerts are the answer.
Sign up now
This month sees the following articles in Catalysis Science & Technology that are in the top ten most accessed:-
Graphene-based materials for catalysis
Bruno F. Machado and Philippe Serp
Catal. Sci. Technol., 2012, 2, 54-75 DOI: 10.1039/C1CY00361E
Conversion of lignocellulose into renewable chemicals by heterogeneous catalysis
Hirokazu Kobayashi , Hidetoshi Ohta and Atsushi Fukuoka
Catal. Sci. Technol., 2012, 2, 869-883 DOI: 10.1039/C2CY00500J
Rational design of heterogeneous catalysts for biodiesel synthesis
Karen Wilson and Adam F. Lee
Catal. Sci. Technol., 2012, 2, 884-897 DOI: 10.1039/C2CY20038D
Nanostructured Titania: the current and future promise of Titania nanotubes
Kevin C. Schwartzenberg and Kimberly A. Gray
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY00538G
Selective oxidation of glycerol to dihydroxyacetone over a Pd–Ag catalyst
Shota Hirasawa , Yoshinao Nakagawa and Keiichi Tomishige
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY20062G
Metal–organic frameworks as heterogeneous catalysts for oxidation reactions
Amarajothi Dhakshinamoorthy , Mercedes Alvaro and Hermenegildo Garcia
Catal. Sci. Technol., 2011, 1, 856-867 DOI: 10.1039/C1CY00068C
Glycerol utilization: solvent-free acetalisation over niobia catalysts
G. S. Nair , E. Adrijanto , A. Alsalme , I. V. Kozhevnikov , D. J. Cooke , D. R. Brown and N. R. Shiju
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY00335J
Shape-controlled synthesis of Cu2O microparticles and their catalytic performances in the Rochow reaction
Zailei Zhang , Hongwei Che , Jiajian Gao , Yingli Wang , Xilin She , Jin Sun , Poernomo Gunawan , Ziyi Zhong and Fabing Su
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY20070H
Metal–organic frameworks for catalysis: the Knoevenagel reaction using zeolite imidazolate framework ZIF-9 as an efficient heterogeneous catalyst
Lien T. L. Nguyen , Ky K. A. Le , Hien X. Truong and Nam T. S. Phan
Catal. Sci. Technol., 2012, 2, 521-528 DOI: 10.1039/C1CY00386K
Fischer–Tropsch reaction–diffusion in a cobalt catalyst particle: aspects of activity and selectivity for a variable chain growth probability
David Vervloet , Freek Kapteijn , John Nijenhuis and J. Ruud van Ommen
Catal. Sci. Technol., 2012, Advance Article DOI: 10.1039/C2CY20060K
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Catalysis Science & Technology? Then why not submit to us today or alternatively email us your suggestions.
Recovering homogeneous catalysts at the end of a chemical reaction can be tricky as the catalysts are in the same phase as the products. But scientists in Canada have now found a way to do this that doesn’t suffer from the slow reaction rates that affect current catalyst recovery systems. Currently, catalyst recovery systems in use in industry rely on an aqueous/organic mixture. The catalyst is dissolved in the aqueous phase and the reagents are in the organic phase. The problem with this, though, is that because the catalyst and reagents only meet at the interface between the two, the reaction is slow.
Philip Jessop and colleagues at Queen’s University, Ontario, have come up with a solvent system that switches from a single phase for a quick reaction to two phases for quick and easy separation. The team tested their system on a homogeneous catalytic reaction. First, they carried out the reaction in a one-phase switchable water/organic solvent mix and then switched the water’s properties to get two phases – one holding the product and the other holding the catalyst. ‘Switchable water is a CO2-switchable solvent – its physical properties can be changed by applying or removing CO2, ‘ explains Sean Mercer from Jessop’s group.
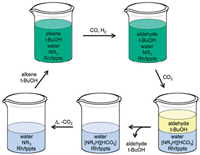
Monophasic hydroformylation and biphasic separation in a liquid mixture of switchable water and tert-butanol
The switchable water solvent mix comprises water and a tertiary amine base, resulting in water free from salts. Introducing CO2 leads to the formation of salts (carbonic acid forms in the water and protonates the amine base, generating charged species or salts), forcing out the organic solvent. The product, which is in the organic solvent, can then be removed, leaving behind the catalyst, which stays in the water. Removing the CO2 by heating and flushing with air causes the charged species to revert back to their original uncharged form, making the water salt-free once more. ‘Fresh reagents and organic solvent can then be added and the reaction can be run again and again,’ says Mercer.
‘Others have done this in a slightly different way, in which the originally hydrophobic catalyst switches into water on passing CO2, but Jessop’s “switchable water” approach has the advantage that he can use simple water soluble ligands that can be bought off the shelf, whilst the ligand switching requires specially designed ligands that are difficult to make,’ says David Cole-Hamilton, an expert in homogeneous catalysis at the University of St Andrews, UK. However, he does point out that there are still problems to be addressed, including a fall off in conversion after several cycles, which he says can almost certainly be fixed by improved reactor and recycler design and by the rigorous exclusion of air.
Another issue, adds Mercer, is that they perform the catalysis in a highly basic medium, so certain reactions can’t be performed. ‘We also need to enlarge the number of reactions that can be performed using this solvent system, as we only demonstrated the hydroformylation of alkenes to aldehydes,’ he says. ‘A second issue is we sometimes observe slight leaching of our precious metal catalyst into the organic phase so it is lost from the process. In the immediate future, we need to find catalysts that leach less, or move to less expensive metals so that losses aren’t as detrimental monetarily.’
Written by Elinor Richards
Recycling of a homogeneous catalyst using switchable water
Sean M. Mercer, Tobias Robert, Daniel V. Dixon and Philip G. Jessop
DOI: 10.1039/C2CY20095C
Kurt Faber and colleagues from the Unviersity of Graz report the reductive dehalogenation of β-haloacrylic ester derivatives using members of the ‘Old Yellow Enzyme’ family of flavoproteins in this HOT Catalysis Science & Technology communication. They have combined the ‘reductive activity of ene-reductases with the spontaneous β-elimination of hydohalous acid from the unstable (saturated) intermediates’ in this biodegradation pathway. Such work is important for its application to the disposal of organic halogenated materials that have negative effects on the environment.
This is another fantastic article that is due to be included in our upcoming Biocatalysis themed issue. Download the communication today – it’s free…
Reductive dehalogenation of β-haloacrylic ester derivatives mediated by ene-reductases
Gábor Tasnádi, Christoph K. Winkler, Dorina Clay, Mélanie Hall and Kurt Faber
Catal. Sci. Technol., 2012
DOI: 10.1039/C2CY20079A
You might also find the group’s previous Dalton Transactions paper interesting:
Bioreduction of α-methylcinnamaldehyde derivatives: chemo-enzymatic asymmetric synthesis of Lilial™ and Helional™
Clemens Stueckler, Nicole J. Mueller, Christoph K. Winkler, Silvia M. Glueck, Karl Gruber, Georg Steinkellner and Kurt Faber
Dalton Trans., 2010, 39, 8472-8476
DOI: 10.1039/C002971H
From themed issue Bridging the gap in catalysis via multidisciplinary approaches
 Heteroaromatic frameworks are valuable products with applications in a range of industries, the largest being in the pharmaceutical and drug discovery business. Developing the synthetic processes used to create these products is a growing field of research and enantioselective organocatalysis is proving to be an interesting and economic route to accomplish this.
Heteroaromatic frameworks are valuable products with applications in a range of industries, the largest being in the pharmaceutical and drug discovery business. Developing the synthetic processes used to create these products is a growing field of research and enantioselective organocatalysis is proving to be an interesting and economic route to accomplish this.
This Perspective article from Karl Jørgensen’s group details a recently developed one-pot synthesis for constructing hydroxyalkyl- and aminoalkyl-substituted heteroaromatic compounds, these optically active products are formed in good yields with high enantioselectivities and minimal waste. Their novel methodology provides an important new route to synthesising heteroaromatic compounds for academia and industry alike.
l
l
Registration to download all articles published in Catalysis Science & Technology is free and provides access to all previously published articles in the journal – sign up here.
Organocatalytic synthesis of optically active heteroaromatic compounds
Łukasz Albrecht , Lars Krogager Ransborg and Karl Anker Jørgensen
DOI: 10.1039/C2CY20101A
You can also follow us on twitter, like us on facebook and sign up to our e-alerts! If you enjoy reading Catalysis Science & Technology, recommend it to your librarian so you can make sure you are subscribed next year.
We welcome submissions for our upcoming Themed Issue ‘Heterogeneous catalytic aerobic oxidation for the synthesis of fine chemicals’, Guest Edited by Professor Alfons Baiker and Dr Tamas Mallat.
 This special issue will focus on novel aspects of aerobic oxidation such as biomass conversion, new catalyst formulations, mechanistic and kinetic studies, application of neoteric solvents such as ionic liquids and supercritical fluids, and reactor development. The scope of the Themed Issue will mainly cover heterogeneously catalyzed oxidation processes but will also include related processes based on homogeneous and biocatalysts.
This special issue will focus on novel aspects of aerobic oxidation such as biomass conversion, new catalyst formulations, mechanistic and kinetic studies, application of neoteric solvents such as ionic liquids and supercritical fluids, and reactor development. The scope of the Themed Issue will mainly cover heterogeneously catalyzed oxidation processes but will also include related processes based on homogeneous and biocatalysts.
Want to submit?
The Themed Issue is due to be published early 2013 and we hope to receive manuscripts by 1st July 2012, we are accepting all manuscript types including Perspective review articles, Minireviews and original research papers as communications or full papers (check our author guidelines for more details). You can submit your work as usual using our online submission system, although please indicate that you intend your paper to be included in this special themed issue.
All manuscripts will undergo the usual rigorous peer-review process to maintain the high quality of the journal and inclusion in the Themed Issue will be at the discretion of the Guest Editors.
Please don’t hesitate to contact us if you have any questions or would like any further information about this special issue.
Isotopic labelling is an important tool to investigate chemical processes, from discrete reactions to pharmacodynamics and biological metabolism. Replacing atoms with isotopes is an interesting area of chemistry and this Hot Communication by Matthew Truppo et al. from Merck is no exception.
The team use transaminases to create chiral amines. The method allows efficient generation of both deuterium and tritium labelled amines and is effective on several ketone substrates, potentially providing a general route to D and T labelled chiral amines for a range of applications.
Asymmetric, biocatalytic labeled compound synthesis using transaminases
Matthew Truppo, Jacob M. Janey, Brendan Grau, Krista Morley, Scott Pollack, Greg Hughes and Ian Davies
This article is due to be published in our upcoming themed issue focusing on biocatalysis, other articles to be included in this special issue include,
Mutational analysis of phenolic acid decarboxylase from Bacillus subtilis (BsPAD), which converts bio-derived phenolic acids to styrene derivatives
Annika Frank, William Eborall, Ralph Hyde, Sam Hart, Johan P. Turkenburg and Gideon Grogan
Stereoselective synthesis of bulky 1,2-diols with alcohol dehydrogenases
Justyna Kulig, Robert Christian Simon, Christopher Rose, Masood Husain, Matthias Häckh, Steffen Lüdeke, Kirsten Zeitler, Wolfgang Kroutil, M Pohl and Dörte Rother
Asymmetric reduction of a key intermediate of eslicarbazepine acetate using whole cell biotransformation in a biphasic medium
Manpreet Singh, Sawraj Singh, Sateesh Deshaboina, Hare Krishnen, Richard Lloyd, Karen Holt-Tiffin, Apurba Bhattacharya and Rakeshwar Bandichhor
C2CY00537A
Scientists from the Indian Institute of Technology have investigated the effect of visible light on the Hantzsch reaction – a method by which to produce dihydropyridines which have found merit for cardiovascular disease treatment. By doing so, they were able to develop a photocatalytic-based methodology for producing 2-arylpyridines. Whilst such compounds are popular in medicinal chemistry and supramolecular coordination chemistry fields, their synthesis can be troublesome.
Armed with a catalytic amount of [Ru(bpy)3]2+ and a household compact fluorescent lamp, the team synthesised the 2-arylpyridine compound through photocatalytic oxidation of the 1,2-dihydropyridine Hantzsch product.
For further details on the reaction conditions and mechanism, read the the Full Paper:
[Ru(bpy)3]2+ Aided Photocatalytic Synthesis of 2-Arylpyridines via Hantzsch Reaction under the Visible Irradiation and Oxygen Atmosphere
Rajakumar Ananthakrishnan and Sarifuddin Gazi
Make sure that you’re signed up to the Catalysis Science & Technology e-alerts to keep up-to-date with the latest journal content.