 Nitrogen oxides are among the most harmful pollutants produced by the combustion of fossil fuels, cause of euthropication of waters and soil contamination.
Nitrogen oxides are among the most harmful pollutants produced by the combustion of fossil fuels, cause of euthropication of waters and soil contamination.
The reduction of the release of these nitrogen oxides from industrial flue gases and diesel engines into the atmosphere is more than ever a hot topic, as testified by the increasing amount of scientific publications on NOx storage and disposal; some of which appeared in Catalysis Science and Technology last month (here and here, also reviewed in this blog).
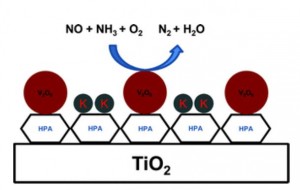
One of the main strategies to convert these oxides into harmless product is the Selective Catalytic Reduction (SCR) that takes place on supported catalysts like those found in exhausts pipes of modern cars. Due to the presence of alkaline metals in biomass derived and fossil fuels that can poison and deactivate them , though, the activity of these catalysts decreases with time; metals like potassium and barium affect the Brønsted acid sites of the catalysts and prevent the ammonia adsorbtion process, essential in the functioning of the system.
A new study by the Danish research group lead by Rasmus Fehrmann addresses this weakness and proposes a possible improvement to the process; the group postulated that in the presence of a super-acidic support for the catalyst, the alkali would preferentially react with the support, leaving the catalytic species untouched.
The super-acid of choice was a member of the heteropoly acids family, a class of solid acids composed of clusters of hydrogen, oxigen and transition metals coordinated around elements of the p-block like silicon, phosphorus or arsenic.
The chosen heteropoly acids, among which the 12-tungstophoshporic acid (TPA), were added to the titanium oxide support in conjunction with V2O6 as the active catalyst and tested in the NH3 promoted SCR. Traditional V2O6/TiO2 and mixed V2O6-WO3/TiO2 catalysts were used as a reference during the activity tests.
After doping with a source of potassium, the catalysts were tested against their pristine counterparts to measure the difference in activity, resulting in a superior performance of the super-acid supports which retained up to 88% of the original activity compared to 33% of the untreated ones.
Find out about these promising catalysts here.
Heteropoly acid promoted V2O5/TiO2 catalysts for NO abatement with ammonia in alkali containing flue gases
Siva Sankar Reddy Putluru, Anker Degn Jensen, Anders Riisager and Rasmus Fehrmann
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C1CY00081K, Paper











 Catalysis Science & Technology is celebrating Easter by highlighting all recently published review articles.
Catalysis Science & Technology is celebrating Easter by highlighting all recently published review articles.