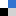In this Chem. Soc. Rev. review article, Kenji Kobayashi and Masamichi Yamanaka from Shizuoka University, Japan, give a detailed account of the host guest chemistry of a particular type of calixarene. Simple calixarenes are usually derived from, for example, a phenol and formaldehyde in an electrophilic aromatic substitution reaction. This yields a puckered basket structure containing a ring of methylene spaced phenols groups.
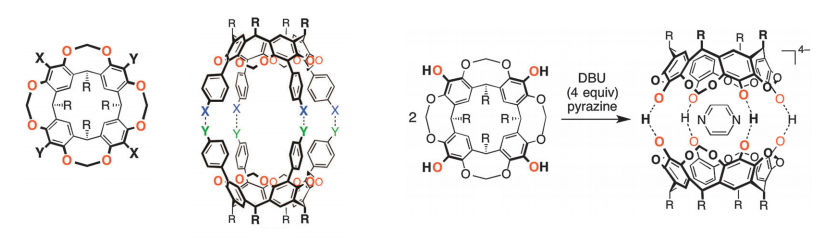
Calixarenes based on 1,3-dihydroxybenzene, also known as resorcinol (calix[4]resorcinarenes) are derived from similar chemistry and are described in this review. Capsule like materials containing covalently bound spacers between pairs of calix[4]resorcinarene units are briefly mentioned, while the majority of the review is devoted to capsules (or cavitands) containing substitutents capable of hydrogen bonding, metal coordination and other dynamic bonding, resulting in powerful, self-assembled host-guest interactions. The calix-[4]-resorcinarenes have extra rigidity in their structure, due to methylene bridging between the resorcinol oxygens of neighbouring units, with functional groups at the in-between 2-position.
Functionality introduced includes carboxylic acids, nitriles, halides, boronic acids, aldehdyes, alcohols and even a biypridyl unit. For example, a cavitand, containing phenol groups, not involved with other bonding, can be deprotonated to neatly bind pyrazine, in a hydrogen bound network, resulting in a dimeric self assembled cavitand.
Generic calix[4resorcinarene structures and a host-guest interaction with pyrazine]
Other examples are provided with mixed donors such as phenols and pyrdine groups in the same material. These heterodimeric cavitands can give access to a more advanced level of specificity, in these cases, for a variety of organic guests. Stronger bound capsule systems are described based on metal coordination. A nitrile substituted calix-[4]-resorcinarene and its palladium, platinum and counter-anion host-guest chemistry are briefly described, while examples self-assembled capsules with coordinating bipyridyl and dithiocarbamate units are also included.
The molecular and synthetic variety in this review is impressive. It is an enjoyable read, and should prove valuable to researchers in many disciplines. The application of such designed and potentially specific calix[4]resorcinarenes, and their mode of action in self-assembling and modifying guest reactivity is a fascinating research area!
Read this Chem. Soc. Rev., Review Article today for free:
Self-assembled capsules based on tetrafunctionalized calix[4]resorcinarene cavitands
Kenji Kobayashi and Masamichi Yamanaka
DOI: 10.1039/C4CS00153B
*Access is free untill the 31.07.14 through a registered RSC account – click here to register