The Wittig reaction was first reported in 1954 and awarded the Nobel Prize in 1979. Today, it is widely used for the preparation of alkenes by reaction of a carbonyl compound with a phosphonium ylide. Despite its age and broad utility, differing mechanisms for the Wittig reaction still feature in the literature and textbooks.
Peter Byrne and Declan Gilheany at University College Dublin have written a substantial review of this transformation, which provides definitive evidence regarding the mechanism of lithium salt-free Wittig reactions of phosphonium ylides.
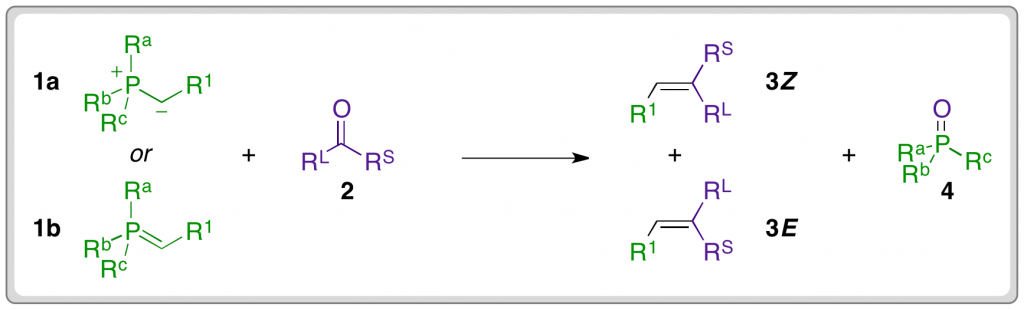
Phosphonium ylides can be represented in either ylide (1a) or ylene (1b) form and they are classified according to their substitution at the α-carbon. The P–C bond of a phosphonium ylide is heavily polarised towards carbon and so ‘R’ groups that offer a greater degree of conjugative stabilisation increase the overall stabilisation of the ylide. Additionally, the nature of the ‘R’ group influences the selectivity for formation of Z or E alkenes.
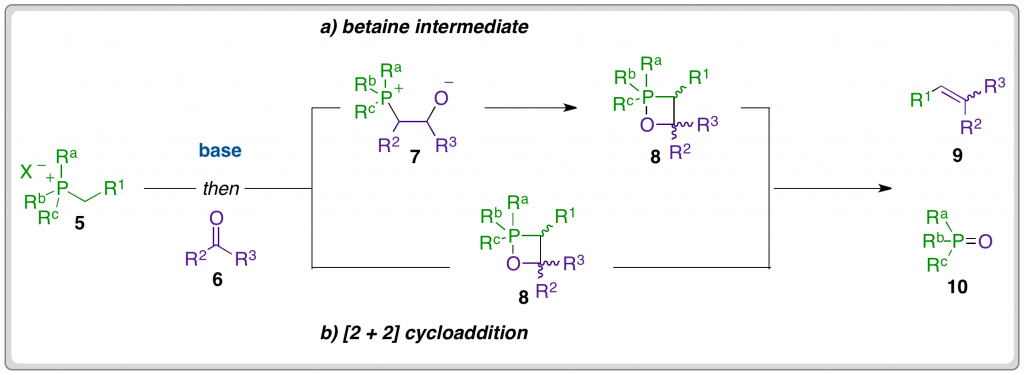
The mechanism that features in many undergraduate textbooks involves the attack of the carbonyl carbon by the nucleophilic ylide α-carbon to form a betaine intermediate (7), which undergoes ring closure to form an oxaphosphetane (OPA) (8) and subsequently decomposes to form the product alkene (9) and a phosphine oxide (10) by-product.
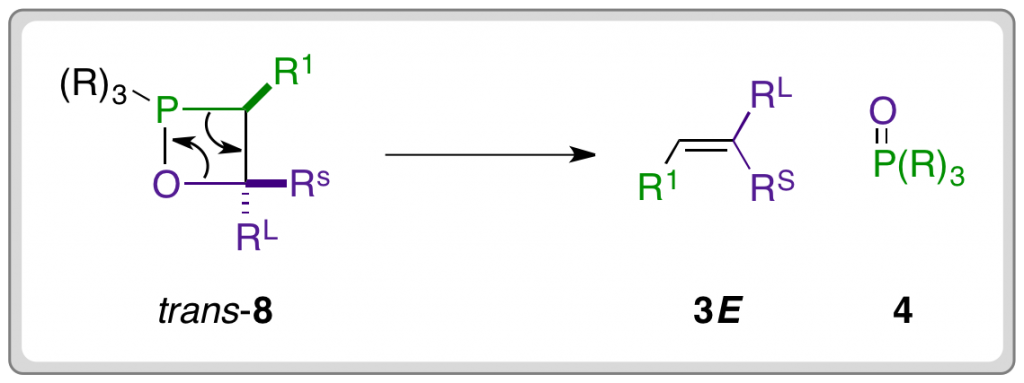
Byrne and Gilheany present a body of evidence that disputes this mechanistic pathway and instead supports an irreversible [2 + 2] cycloaddition between the ylide and carbonyl compound to directly form the OPA (8). The OPA then decomposes in a stereospecific manner, meaning that the stereochemistry of the alkene product is determined by the shape of the transition state for the [2 + 2] cycloaddition, and hence the structure of the OPA. The E-selectivity commonly observed in the case of semi-stabilised and stabilised ylides is explained by a kinetic preference for the formation of trans-OPA, which decomposes to form the E-alkene.
This clarification of the mechanism for the lithium salt-free Wittig reaction will likely feature in future editions of undergraduate textbooks and will encourage many chemistry lecturers to update their course notes before the start of the new academic year.
For more, read this HOT Chem Soc Rev Review Article in full:
The Modern Interpretation of the Wittig Reaction Mechanism
Peter A. Byrne and Declan G. Gilheany
Chem. Soc. Rev., 2013, Advance Article
DOI: 10.1039/C3CS60105F
Alice Williamson is a guest web-writer for Chem Soc Rev. She is currently a postdoc for the OSDDMalaria Project in Dr. Matthew H Todd’s group at the University of Sydney.