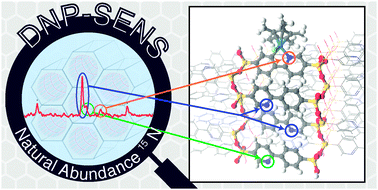
In dynamic nuclear polarisation surface enhanced NMR spectroscopy (DNP SENS), a porous or particulate sample is wetted with a radical solution. The large polarisation of the radicals’ unpaired electrons is then transferred to surrounding nuclear spins, with a typical signal enhancement of between 10 and 100. This can decrease experimental time dramatically, whilst probing specifically the surface functionality.
In their recent communication in PCCP, the research groups of Professor Lyndon Emsley and Professor Christophe Copéret collaborated to characterise the organic part of a periodic mesoporous organosilicate (PMO). Structural changes following functionalisation with an organoiridium compound were studied using DNP SENS. Remarkably, 15N (0.37% natural abundance) DNP SENS spectra revealed the appearance of a new chemical environment following functionalisation, corresponding to nitrogen atoms (in the PMO) bonded to Iridium (III). This key piece of evidence allowed the authors to elucidate a layered structure in which only the surface layers were available for functionalisation.
Whilst the 15N spectra would have taken weeks to acquire using conventional NMR methods, DNP SENS experiments took only a matter of hours, highlighting the power of this fascinating method.
Full details can be found in the PCCP communication:
Molecular-level characterization of the structure and the surface chemistry of periodic mesoporous organosilicates using DNP-surface enhanced NMR spectroscopy
Wolfram R. Grüning, Aaron J. Rossini, Alexandre Zagdoun, David Gajan, Anne Lesage, Lyndon Emsley and Christophe Copéret
DOI: 10.1039/C3CP00026E
By Alexander Forse











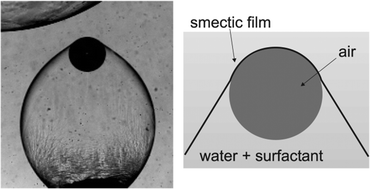 Sometimes you just have to sit back, read, and enjoy the ride. This is exactly the case with the work of Kirsten Harth and
Sometimes you just have to sit back, read, and enjoy the ride. This is exactly the case with the work of Kirsten Harth and  In my understanding, science is the search for answers. The validity of the research is then defined by the nature of the question.
In my understanding, science is the search for answers. The validity of the research is then defined by the nature of the question. 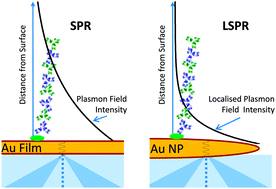 Technology based on surface plasmon resonances and localised surface plasmon resonances is surging. And so is the research into SPR effects, see for instance the recent PCCP themed collection:
Technology based on surface plasmon resonances and localised surface plasmon resonances is surging. And so is the research into SPR effects, see for instance the recent PCCP themed collection: 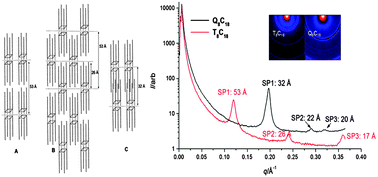

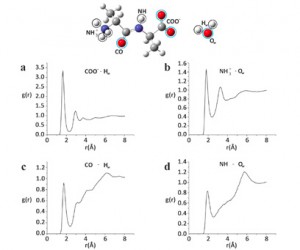
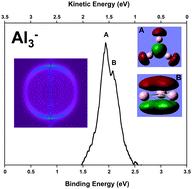 Between bulk metals and their individual atoms lies the murky world of metallic clusters. These often have unique properties, and understanding them is as challenging as it is interesting. Many properties are a direct function of cluster size, and provide important insights into the progression from individual atoms to bulk solids. It is therefore important to ascertain the exact size at which a particle ceases to be classified as a cluster and becomes a bulk solid. This moment can either be measured experimentally or calculated using a theoretical model.
Between bulk metals and their individual atoms lies the murky world of metallic clusters. These often have unique properties, and understanding them is as challenging as it is interesting. Many properties are a direct function of cluster size, and provide important insights into the progression from individual atoms to bulk solids. It is therefore important to ascertain the exact size at which a particle ceases to be classified as a cluster and becomes a bulk solid. This moment can either be measured experimentally or calculated using a theoretical model.