 Gold has been revered by humanity for millennia as a decorative expression of status and a way to measure monetary wealth. It can be found pure, it doesn’t tarnish with use, and is easily worked. So far so good, and gold is still valued for those exact properties today. However, from a chemical perspective it was long considered inert, and other rarer, more expensively extracted metals became the darlings of catalysis. More recently, in a similar manner to the development of noble gas chemistry, there has been a surge of discovery and development in the uses of gold as a catalytic material.
Gold has been revered by humanity for millennia as a decorative expression of status and a way to measure monetary wealth. It can be found pure, it doesn’t tarnish with use, and is easily worked. So far so good, and gold is still valued for those exact properties today. However, from a chemical perspective it was long considered inert, and other rarer, more expensively extracted metals became the darlings of catalysis. More recently, in a similar manner to the development of noble gas chemistry, there has been a surge of discovery and development in the uses of gold as a catalytic material.
Rodriguez and Koper provide a fascinating review of the surprising catalytic properties that have been discovered over the last few decades, with a focus on electrocatalysis. They gather up various findings from all over the world, consolidate them and tease out the recurrent themes. It seems that gold catalysts are not easily poisoned, which can only be a good thing, but can be very choosy about the types of reactions they will catalyse, with different surfaces even having different catalytic preferences. All this suggests an electrocatalyst with the potential to be highly tuneable. If that pricks your curiosity then I strongly suggest that you read this perspective article, which surely forms a useful basis for future investigations.
Electrocatalysis on gold
Paramaconi Rodriguez and Marc T. M. Koper
Phys. Chem. Chem. Phys., 2014, Advance Article
DOI: 10.1039/C4CP00394B












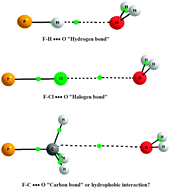 The work of PCCP Advisory Board member
The work of PCCP Advisory Board member 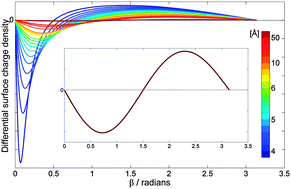 The results of their study are quite striking. They found that by varying the distance between the fullerene and the point charge, the surface charge density of C60 can be manipulated. In other words, they predict that one could selectively polarise a fullerene molecule to a greater or lesser extent. They were also able to predict the separation parameters necessary in order to achieve this, including identifying the tipping point at which the charge distribution is expressed as a purely positive hemisphere and a purely negative hemisphere.
The results of their study are quite striking. They found that by varying the distance between the fullerene and the point charge, the surface charge density of C60 can be manipulated. In other words, they predict that one could selectively polarise a fullerene molecule to a greater or lesser extent. They were also able to predict the separation parameters necessary in order to achieve this, including identifying the tipping point at which the charge distribution is expressed as a purely positive hemisphere and a purely negative hemisphere.