Elangannam Arunan and Devendra Mani of the Indian Institute of Science have investigated an interesting C···Y bond, a “carbon bond”, which occurs when one of the hydrogen atoms of methane is replaced by an electron withdrawing group.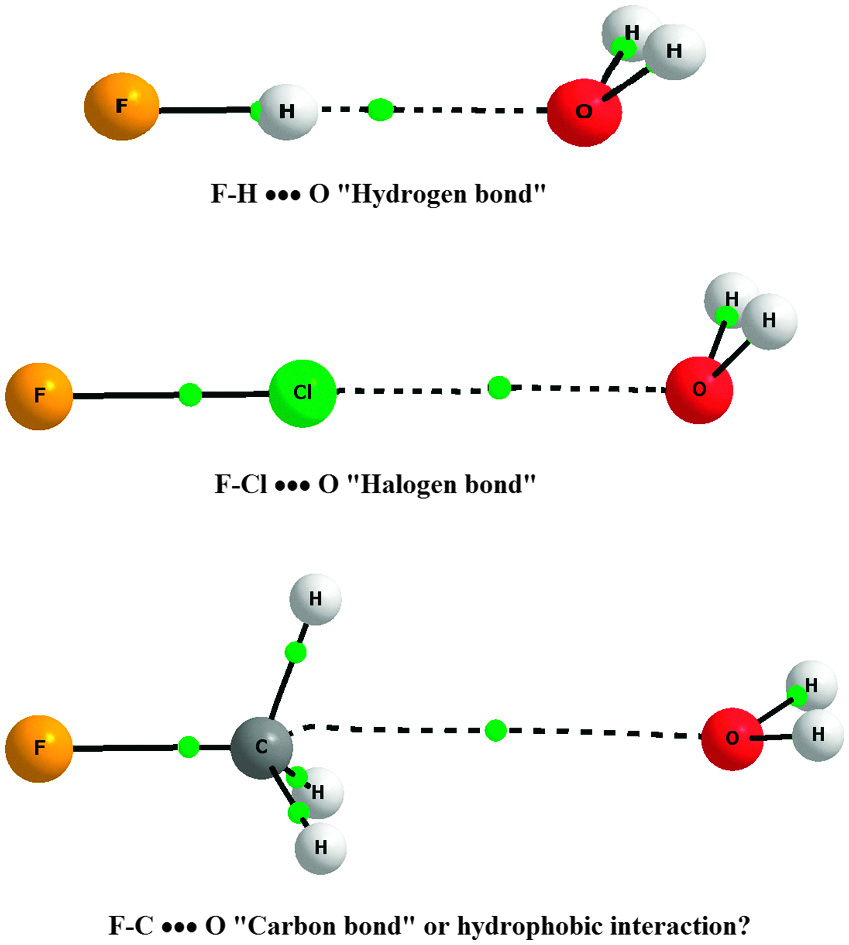
Weak interactions are very important in molecules of life, such as water and DNA, in supramolecular chemistry and in crystal design and engineering. Traditionally, these interactions were classified as hydrogen bonding and van der Waals interactions, but in recent decades, other weak interactions, such as halogen bonds, chalcogen bonds and pnicogen bonds, have been investigated and classified. An interesting question is whether carbon atoms can also play a role in weak interactions, in addition to the more electronegative elements known to take part.
When one of the hydrogen atoms in methane is replaced with an electron withdrawing group, such as -OH or –F, the CH3 tetrahedral face becomes a positive centre. Using NBO analysis and vibrational frequency data, Arunan and Mani showed that this positive centre could accept electron density from atoms like O in water, giving rise to a novel C···Y bond, which could be called a carbon bond.
Arunan says that given the abundance of alkyl groups in biological systems, such carbon bonding interactions could play a significant role in biology, which has yet to be recognised. “Hydrogen bonds are just sufficiently strong and can be broken and made under ambient conditions, helping life. Carbon bonds are weak, and if they were not much of what we know about life could not be.”
For more details, read their article:
The X-C•••Y (X=O/F, Y=O/S/F/Cl/Br/N/P) ‘carbon bond’ and hydrophobic interactions
Devendra Mani and E Arunan
DOI: 10.1039/C3CP51658J










