Scientists from the USA provide important insights into the complex mechanism of amyloid-induced membrane disruption in their recent PCCP article, which will be included in our soon-to-be-published themed issue on biophysical studies of protein misfolding and amyloid diseases.
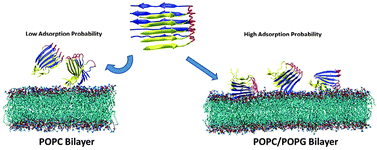
Zheng et al. investigated the adsorption, orientation and suface interaction of the p3 pentamer with lipid bilayers composed of both pure zwitterionic POPC and mixed anionic POPC-POPG lipids using molecular dynamics simulations.
A pathological hall-mark of Alzheimer’s disease is the co-existence of extracellular plaques of amyloid-β and intracellular tangles of tau protein in the brain. It is generally thought that the interactions of Aβ oligomers with neuronal membranes are responsible for brain cell death and subsequent damage to learning and memory functions, but the exact details of the process are unclear. This work is valuable for future development of therapeutic agents and approactes to disrupt Aβ adsorption, aggregation and surface interaction on the membrane.
Read this HOT article today:
Molecular interactions of Alzheimer amyloid-β oligomers with neutral and negatively charged lipid bilayers
Xiang Yu, Qiuming Wang, Qingfen Pan, Feimeng Zhou and Jie Zheng
DOI: 10.1039/C3CP44448A
You may be interested to see our online collection of biophysics and biophysical chemistry in PCCP.