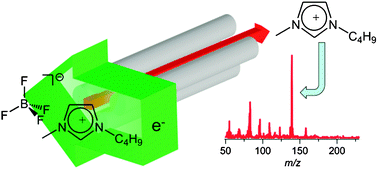
The authors found that the ILs evaporated as neutral ion pairs.
Read this exciting research in PCCP:
The vapour of imidazolium-based ionic liquids: a mass spectrometry study
A. Deyko, K. R. J. Lovelock, P. Licence and R. G. Jones
Phys. Chem. Chem. Phys., 2011
DOI: 10.1039/C1CP21821B










