Edmond W.K. Young, Aaron R. Wheeler and Craig A. Simmons.
University of Toronto, Canada.
Why is this useful?
In many microfluidics applications, the presence of bubbles can be undesirable because of their tendency to disturb fluid flow in microchannels. For cell culture studies in particular, cells that are not strongly adhered to the underlying substrate can be sheared, detached, and entrained by passing bubbles, leaving behind regions depleted of cells. Many microchannel designs therefore incorporate either bubble traps [1] or complex valve configurations [2] to eliminate bubbles from being introduced into the channels. However, these elements add complexity, and thus are not ideal for use with rapid prototyping experiments, in which fluid is typically delivered by means of a syringe. We present a tip that avoids the common phenomenon of bubble injection by careful attention to reservoir fluid volumes.
What do I need?
- PDMS-glass microchannel, irreversibly sealed, with polyethylene tubing (Intramedic) inserted into cored-out holes in the PDMS and sealed with epoxy (LePage)
- Syringe, 1 mL (BD)
- Syringe needle, 18 gauge (BD)
- Fluid mediumTypical Microfluidic Chip
Figure 1. A Typical Microfluidic Chip
What do I do?
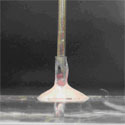
Prior to sample injection, the microfluidic device is primed with fluid and the inlet and outlet ports are not completely filled (Figure 1). If a syringe needle is inserted into the inlet port (Figure 2a) at this stage, air will be trapped between the syringe needle and the liquid-air interface, causing bubbles to be injected into the microchannel. To avoid this phenomenon:
1. Insert syringe needle into outlet port (Figure 2b). Note that this traps a bubble on the outlet side.
2. Slowly depress syringe to push fluid toward the inlet side. Do so until fluid reaches the top of the inlet port and forms a small bead.
3. Remove syringe needle from outlet port. Note that the liquid interface on the outlet side should now be lower than before Step 2. However, the trapped bubble from Step 1 should now be eliminated.
4. Depress syringe to generate a small droplet at the tip of the needle (Figure 2c).
5. Touch the droplet at the needle tip to the bead of fluid at the inlet (Figure 2d). Merging the droplet and the bead prevents formation of an air bubble.
6. Inject sample from syringe into channel as desired; no bubbles will be injected.
References
[1] E. Leclerc, Y. Sakai, and T. Fujii, Cell culture in 3-dimensional microfluidic structure of PDMS (polydimethylsiloxane). Biomed. Microdevices, 2003, 5(2), 109-114.
[2] L. Kim, M.D. Vahey, H.-Y. Lee, and J. Voldman, Microfluidic arrays for logarithmically perfused embryonic stem cell culture. Lab Chip, 2006, 6, 394-406