Posted on behalf of Gwenda Kyd, web-writer for CrystEngComm
Cuprous oxide (Cu2O) has many potentially useful applications resulting from its optical and magnetic properties. It has a small band-gap, which gives it attractive photocatalytic behaviour, further enhanced by its low cost and environmental acceptability. However, the control of size and morphology of Cu2O particles can be problematic.
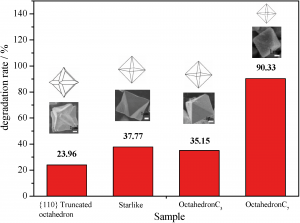
A new paper demonstrates use of a simple wet-chemical method to form a new class of Cu2O particles existing as short hexapods, octahedra or {110} truncated octahedra. These are formed in a size- and morphology-controllable manner depending on the reaction conditions. The degradation of methyl oxide (MO) dye gives a measure of photocatalytic activity of the different morphology crystals (see diagram below). The results indicate that activity varies with morphology, with the best results obtained for smallest-sized octahedra and potential use in the treatment of organic pollutants is indicated.
For more details see the paper at:
The morphology dependence of cuprous oxide and its photocatalytic properties
Ru Li, Xuefeng Yan, Liangmin Yu, Zhiming Zhang, Qunwei Tang and Yongping Pan
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41470A
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently she works as a scientific database editor.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently she works as a scientific database editor.