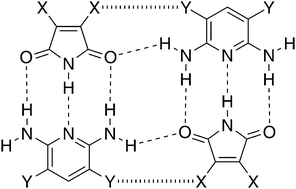
The team chose systems of a series of crystals and co-crystals formed by 3,4-diX-substituted maleimide, 3,5-diYsubstituted-2,6-diaminopyridine, where X, Y = H, Cl, Br, and some of their 1 : 1 adducts.
Hydrogen bonding continues to play a central role in crystal engineering strategies but other interactions have received increasing attention as supramolecular synthons. Among these, halogen bonding is a stabilizing directional interaction between the halogen atom and an electron donor.
Read the article for free until 12th May 2011 here.
Structures of hydro-, chloro-, and bromo-substituted maleimides and 2,6-diaminopyridines, and of some of their 1:1 heterodimers
Tullio Pilati and Franco Cozzi
CrystEngComm, 2011, Advance Article, DOI: 10.1039/C1CE05166K
 You might also find interesting a recent Dalton Transactions blog post called “IUPAC define the hydrogen bond“.
You might also find interesting a recent Dalton Transactions blog post called “IUPAC define the hydrogen bond“.
Why not check it out here.










