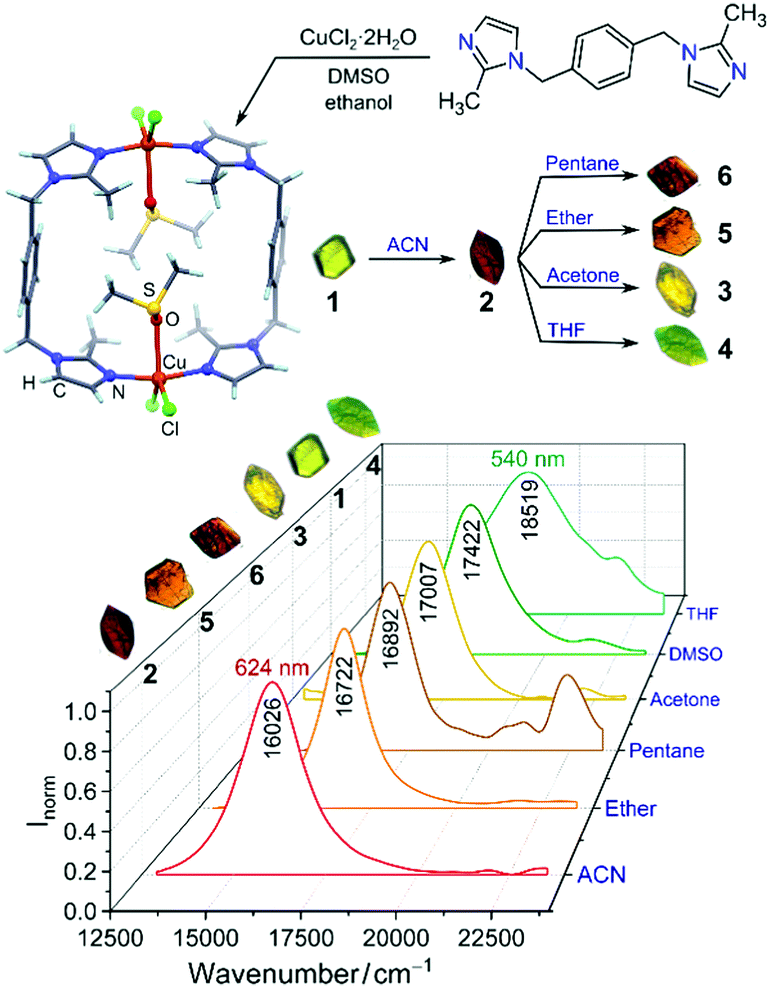
Mohamed Eddaoudi and co-workers at KAUST have synthesised a porous metal organic framework (MOF) constructed from carboxylic acid-functionalised imidazole linkers coordinated to yttrium and potassium cations. The researchers classified this material as a zeolite-like MOF (ZMOF) due to its topological resemblance to the naturally occurring zeolite mineral analcime.
The material’s architecture, with cylindrical channels and a pore aperture measuring 3.8 x 6.2 Å, promised utility as a molecular sieve, and the authors showed the ZMOF could be used to sort small chain alkanes based on their level of branching. Linear and mono-branched pentanes and butanes were adsorbed by the material for different lengths of time (linear isomers were retained longer than their branched counterparts) allowing kinetic separation, while the di-branched alkane 2,2,4-trimethylpentane was excluded entirely. The authors anticipate that this material could have practical applications in crude oil refining, to upgrade petroleum into more energy-efficient fuels with reduced emissions.
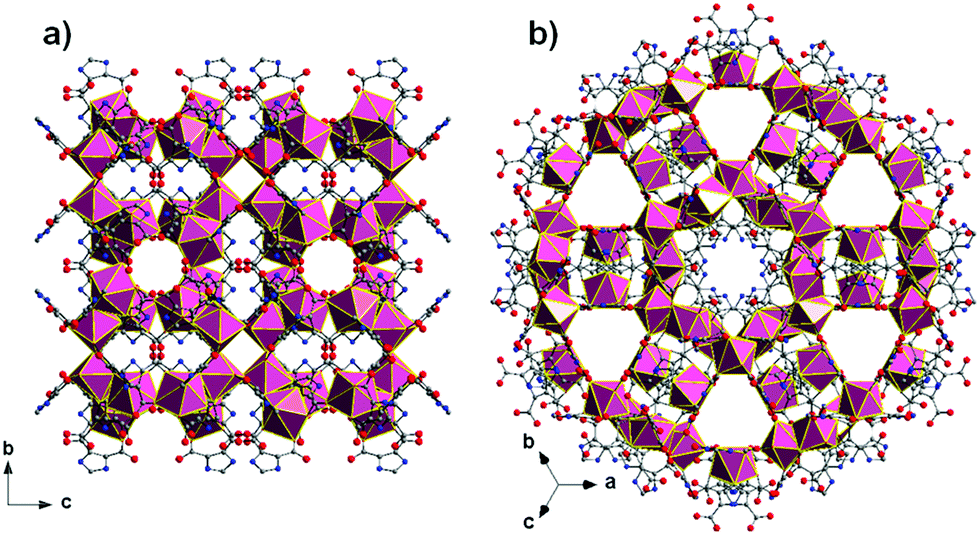
ZMOF crystal structure with analcime (ana) topology showing channels and pore aperture.
The petroleum used to power internal combustion engines consists of a mixture of low molecular weight, linear and branched alkanes. The research octane number (RON) is a standard measure of petroleum performance, and indicates how much pressure a fuel can withstand before self-igniting (knocking) in the engine. High compression engines, which are more energy efficient and release less emissions than regular engines, require high RON fuels.
The RON increases with the proportion of branched alkanes, so can be improved by supplementing fuels with branched isomers obtained by catalytic isomerisation. This process generates a mixture of linear and branched alkanes, so the desired products must be isolated via fractional distillation, which is energy intensive. This creates a dilemma: high RON fuels are more energy efficient, but their energy-intensive production reduces the net benefit.
The authors envisaged an energy-efficient strategy for increasing the RON of petroleum fuels: A low RON fuel is pumped into the engine, where it encounters a separation chamber consisting of ZMOF-based membranes. The membrane excludes and redirects di-branched alkanes, which have a very high RON, to the internal combustion engine. The low RON fraction, consisting of mono-branched and linear alkanes, passes through the ZMOF pores to undergo further reforming processes downstream. In other words: low RON fuels go in, but high RON fuels are combusted.
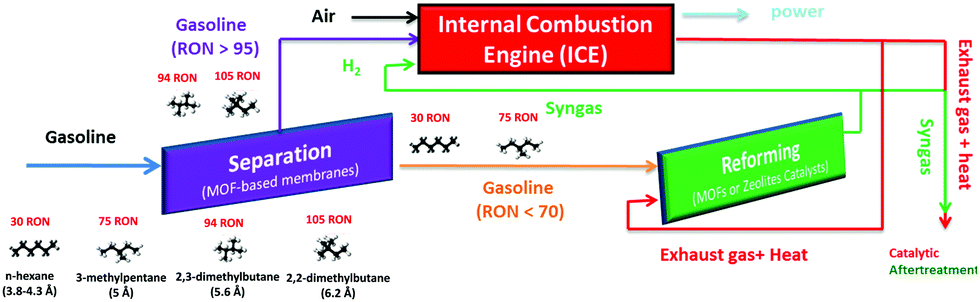
Scheme showing the RON of common small-chain alkanes and the use of ZMOF membranes in upgrading gasoline by separating alkanes based on their level of branching
In this work the authors show the potential of ZMOFs to maximise the energetic potential and reduce emissions of petroleum based fuels, while also offering a glimpse of the more general strategy of energy-efficient separations of chemically-similar molecules using tailored materials.
To find out more please read:
Upgrading gasoline to high octane number using zeolite-like metal organic framework molecular sieve with ana-topology
M. Infas H. Mohideen, Youssef Belmabkhout, Prashant M. Bhatt, Aleksander Shkurenko, Zhijie Chen, Karim Adil, Mohamed Eddaoudi.
Chem. Commun., 2018, 54, 9414-9417
DOI: 10.1039/c8cc04824j
 About the author
About the author
Zoë Hearne is a PhD candidate in chemistry at McGill University in Montréal, Canada, under the supervision of Professor Chao-Jun Li. She hails from Canberra, Australia, where she completed her undergraduate degree. Her current research focuses on transition metal catalysis to effect novel transformations, and out of the lab she is an enthusiastic chemistry tutor and science communicator.














![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) Ge
Ge![[double bond splayed right]](https://www.rsc.org/images/entities/h2_char_e00a.gif) double bond
double bond




 About the author
About the author