An efficient iron oxide catalysed cross-coupling reaction between organometallic species and cyclic ethers, via activation of C(sp3)-H, has been developed by scientists in India. This is the first example of iron oxide mediated direct C-C bond formation without expensive or toxic ligands.
Metal catalysed cross-coupling reactions for C-C bond formation via C-H activation is a hot topic in catalysis research at the moment. Many reactions via the activation of C(sp2)-H have been reported, but examples via C(sp3)-H activation remain much more elusive.
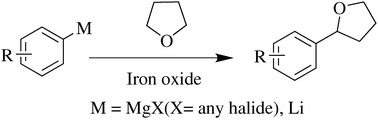 Iron oxide mediated C-C bond formation
|
Ram Vishwakarma and colleagues at the Indian Institute of Integrative Medicine have cleverly devised a cross-coupling reaction that does exactly that. They found that reacting alkyl- and aryl-magnesium halides with tetrahydrofuran (and other cyclic ethers) via activation of the C(sp3)-H results in C-C bond formation. The method is inexpensive (thanks to the cheap iron catalyst) and no toxic ligands are used. |
I’m sure you’ll agree, this is an interesting addition to a hot research area and leaves me wondering about the impact this will have in research laboratories across the globe.
Fancy knowing more about the reaction conditions and substrates used in this reaction? Then download the ChemComm communication, which will be free to access until the 10th June 2011.