Most organic chemists asked to imagine a macrocycle with 19 atoms in the backbone, and with three alkenes and one alkyne, would likely place at least two of the alkenes in conjugation, especially if they were familiar with macrolide antibiotic rifamycin.
Conjugated dienes are usually perceived as most stable; naturally-occurring macrocycles with skipped or non-conjugated dienes are a synthetically challenging class of compounds with biological activity.
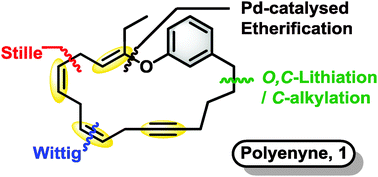
In a recent Chemical Communications article, the research groups of Professor Ian Fairlamb and Professor Richard Taylor describe an elegant regio- and stereoselective synthetic approach to an analogue of 19-membered macrocycle phacelocarpus 2-pyrone A. The target compound is distinctly challenging due to its unique, multiple 1,4-pattern of skipped alkene and alkyne functionality.
Several effective synthetic campaigns of 1,4-diene motifs capitalize on alkene/alkyne metathesis or cross-coupling strategies. However, most methods lack stereoselectivity. In contrast, Fairlamb, Taylor and co-workers achieved the construction of the macrocycle in 6.5% yield over 11 steps in the longest linear route. In doing so, they stereoselectively combined three fragments in the first use of bifunctional (Z)-vinylstannyl-posphonium salt as a nucleophile with stepwise Wittig and Stille reactions.
The successful Z-stereoselectivity of the Wittig reaction was accompanied by the full retention of the Z-vinyl stannane functionality. While stereoselectivity in the Stille cross-coupling was anticipated as problematic due to the sensitive nature of the allylic vinyl ether moiety in the substrate, the authors were pleased that the macrocyclization succeeded with an E:Z ratio of 5:1.
To discover all the synthetic details about the chemistry from the Fairlamb and Taylor groups, read the ChemComm article in full – it’s free to access* until 3rd July:
Macrocyclic polyenynes: a stereoselective route to vinyl-ether-containing skipped diene systems
Thomas O. Ronson, Martin H. H. Voelkel, Richard J. K. Taylor and Ian J. S. Fairlamb
Chem. Commun., 2015, 51, 8034-8036
DOI: 10.1039/C5CC02091C
Also of interest may be the recently published Chemical Science Perspective Article “Macrocycles: lessons from the distant past, recent developments, and future directions” by Organic and Biomolecular Chemistry Editorial Board Chair Professor Andrei K. Yudin (Chem. Sci., 2015, 6, 30-49).
*Access is free through a registered RSC account – click here to register
Dr. Tezcan Guney is a web writer for Chemical Society Reviews, Chemical Science and Chemical Communications. Dr. Guney received his Ph.D. from the Department of Chemistry at Iowa State University with Prof. George Kraus, where he focused on the synthesis of biologically active polycyclic natural products and multifunctional imaging probes. Currently, he is a postdoctoral research scholar at the Memorial Sloan-Kettering Cancer Center in New York with Prof. Derek Tan, contributing to the efforts to access biologically active small molecules using the diversity-oriented synthetic approach.











 Recently, visible light, a sustainable and affordable energy resource, gained substantial interest with its capability to selectively access chiral molecules from prochiral substrates without undesirable by-products. Transformations including aldehyde α-functionalization and [2+2] cycloadditions demonstrate the potential of visible light in the presence of a photosensitizer.
Recently, visible light, a sustainable and affordable energy resource, gained substantial interest with its capability to selectively access chiral molecules from prochiral substrates without undesirable by-products. Transformations including aldehyde α-functionalization and [2+2] cycloadditions demonstrate the potential of visible light in the presence of a photosensitizer.