HSP90 (Heat Shock Protein 90) is a chaperone protein which is involved in the disease pathways of many cancers, and such neurodegenerative illnesses as Alzheimer’s and Parkinson’s disease. The inhibition of HSP90 has gained a great deal of attention since its discovery, and offers the potential to treat many serious illnesses. Much interest has focused on geldanamycin—a benzoquinone ansamycin which is highly effective in the inhibition of HSP90. Unfortunately, geldanamycin suffers from high liver toxicity in addition to poor stability and solubility which greatly limits its therapeutic utility.
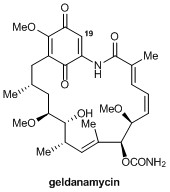
Christopher Moody at the University of Nottingham has devoted much research toward the targeting and inhibition of HSP90. His group recently discovered that the 19-position plays a key role in geldanamycin’s toxicity, and that substitution at that position can render the compound non-toxic, through the suppression of conjugate addition reactions which are thought to be responsible for its hepatotoxicity.
While Moody previously utilized the Stille reaction for substitution at this position, the transformation was limited in cases, not scalable, and its industrial application was hampered by undesirable, toxic reagents and waste products. In this Communication, Moody and Kitson overcome these problems by employing the Suzuki–Miyaura reaction to install functionality at the 19-position. Using a modification of the Suzuki–Miyaura reaction previously described by Eli Lilly researchers, Moody was able to obtain functionalised geldanamycins in yields which compare well with or exceed those obtained by the Stille protocol.
Beginning with readily accessible 19-iodogeldanamycin (1), the cross-coupling reaction allows a range of substituents to be installed easily, using an array of widely available boronic acids and esters. Aryl-, vinyl- and allyl-groups could be installed with excellent yields, while the use of alkyl boronic acids and esters afforded moderate results. The electronic supplementary information contains full details of the reaction optimisation.
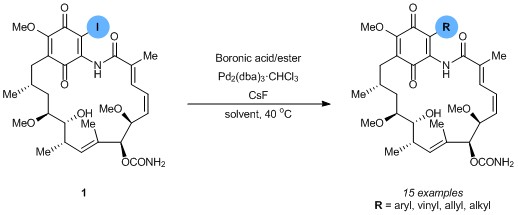
This method allows non-toxic 19-substituted-geldanamycins to be prepared efficiently and without the disadvantages associated with the previous Stille route. Not only will this benefit the synthesis of geldanamycins within the pharmaceutical industry, but it should also encourage further clinical research of these important compounds.
For more, check out the ChemComm article in full:
An improved route to 19-substituted geldanamycins as novel Hsp90 inhibitors – potential therapeutics in cancer and neurodegeneration
Russell R. A. Kitson and Christopher J. Moody
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC43457E
Ruth E. Gilligan is a guest web-writer for ChemComm. She has recently completed her PhD in the group of Prof. Matthew J. Gaunt at the University of Cambridge, focusing on the development and application of C–H functionalisation methodology.










